-
砷(As)在自然环境中分布较为广泛。近年来,由于含As矿物的开采加工和含As废弃物处理不当等原因造成了中国农田土壤中As含量不断上升[1]。据调查统计,中国产生含As矿渣50万t·a−1,已囤积的As渣超过200万t,约有2 000万人生活在土壤As污染高风险区域[2-3]。在从源头进行调控的同时,寻求一种合理且高效的土壤As污染治理方法已迫在眉睫。生物质炭作为一种新兴吸附剂,是将生物质材料置于高温限氧环境中热解后得到的一类稳定的、高度芳香化且含碳丰富的固态物质[4]。生物质炭具有孔隙发达,比表面积较大,阳离子交换性能较强等优点[5],将它作为吸附剂施入土壤后可钝化土壤中重金属,降低其生物有效性[6]。李梦柯等[7]研究发现:施用10%稻壳生物质炭可显著降低土壤中重金属有效态的含量。As(Ⅴ)通常比As(Ⅲ)更容易被吸附在铁介质表面上,当铁处于氧化态时,As(Ⅲ)将被氧化为As(Ⅴ),从而促进As从不稳定态向稳定态转化[8]。将含铁材料施入土壤后可影响土壤pH,进而影响土壤胶体的表面电荷和土壤中As的形态[9-10]。铁改性生物质炭施入土壤后,铁材料在被氧化生成铁氧化物的过程中可以通过共沉淀的方式固定土壤中的As。另外,生物质炭表面存在—OH−、—COOH等含氧官能团,可以与土壤中的As生成非晶态的难溶络合物[11]。胡立琼等[12]将氯化铁(FeCl3)、氯化亚铁(FeCl2)、铁(Fe0)和氧化铁(Fe2O3)等4种含铁材料加入As污染水稻Oryza sativa土中,这4种材料对As均有较好的稳定效果,且以FeCl3效果最好。园林废弃物,即园林植物自然凋落或人工修剪所产生的植物残体,主要有草屑、树叶、乔灌木剪枝和死亡植株等[5]。随着城市园林绿化面积的增加,园林废弃物的产生量也在逐年增加。有学者通过园林废弃物热解工艺将其制备成生物质炭并将其施入土壤,可有效提高土壤有机碳含量,增强土壤保水保肥能力[13],还可以有效吸附土壤中重金属污染物,降低重金属在土壤中的迁移性和生物有效性[14]。目前,已有不少研究利用园林废弃物制备生物质炭进行重金属污染土壤修复[15-17],但将它进行载铁改性作为吸附剂用以固定As(Ⅴ)的研究鲜有报道。本研究以典型园林废弃物法国梧桐Platanus orientalis的修剪枝为原料制备铁改性生物质炭,与土壤混合制备炭土混合物,用于批量吸附试验[16, 18-19],研究施用改性生物质炭后,土壤对溶液中As(Ⅴ)的吸附效果。此外,本研究通过拟合等温吸附模型和动力学吸附模型考察As(Ⅴ)溶液初始质量浓度和吸附时间对吸附效果的影响,初步揭示其吸附机制,以期为生物质炭及铁改性生物质炭在As(Ⅴ)污染土壤治理方面的应用提供依据。
HTML
-
所用土壤采自浙江省杭州市临安区竹林村的一处菜地。根据中国土壤质地分类标准,该土壤为粉砂质黏壤土。将所采土壤于自然条件下风干、剔除碎石及植物根系等杂物,研磨并过2 mm筛备用。
法国梧桐修剪枝由诚邦生态环境股份有限公司提供。将枝条切碎通风晾干至恒量后,使用小型炭化设备(ECO-8-10,湖州宜可欧环保科技有限公司)在限氧条件下热解制备生物质炭。设置热解炉的升温速率为25 ℃·min−1,当温度上升至650 ℃后保持2 h,使样品充分热解制备成生物质炭。将生物质炭研磨后过2.000 mm和0.145 mm筛待用,取一定量过2.000 mm筛,以炭铁质量比为20∶1的比例加入FeCl3溶液中,充分搅拌后将溶液-炭混合物放入超声仪中,在25 ℃条件下超声1 h使其混合均匀;放入恒温烘箱中于65 ℃条件下烘干至恒量;再置于炭化炉中于650 ℃条件下再次热解1 h,得到铁改性生物质炭。将制备完成的铁改性生物质炭研磨过筛后备用。
-
分别将原始生物质炭和铁改性生物质炭以3%的质量分数与供试土壤混合制成炭土混合物[20-22],分别标记为原始生物质炭处理和铁改性生物质炭处理,以不施炭的土壤为对照。
等温吸附试验:以砷酸二氢钠(Na2HAsO4·7H2O)为试剂配置质量浓度为1 000 mg·L−1的As(Ⅴ)溶液,以0.01 mol·L−1的NaCl溶液作为支持电解质。分别称取1.00 g未施炭对照土壤和施炭土壤,投加到25 mL As(Ⅴ)初始质量浓度分别为0、2、5、10、20、40、100、200 mg·L−1的As(Ⅴ)溶液中,调节pH为7.0,于25 ℃恒温摇床中以180 r·min−1振荡24 h,而后在3 500 r·min−1的转速下离心20 min,经0.45 μm尼龙滤膜过滤,取滤液用电感耦合等离子体发射光谱(ICP-OES)测定溶液中As(Ⅴ)的质量浓度。
动力学吸附试验:分别称取1.00 g未施炭对照土壤和施炭土壤,投加到25 mL 40 mg·L−1的As(Ⅴ)溶液中,调节pH至7.0,放入恒温摇床中在25 ℃条件下以180 r·min−1振荡,分别于0.5、1.0、2.0、4.0、8.0、12.0、18.0、24.0 h取出,于3 500 r·min−1的转速下离心20 min,后经0.45 μm尼龙滤膜过滤,取滤液用ICP-OES测定溶液中As(Ⅴ)的质量浓度。
-
供试土壤基本理化性质按照《土壤农业化学分析方法》[23]中的方法测定。土壤砂粒、粉粒和黏粒质量分数分别为19.7%、34.9%和45.4%。根据联合国粮农组织(FAO)的分类体系,该土壤为黏壤土。其有机质质量分数为3.08%,土壤pH为5.14,阳离子交换量为12.9 cmol·kg−1,电导率为0.10 dS·m−1,在该土壤中未检出As(Ⅴ)。供试生物质炭的基本理化性质测定参照YANG等[14]。2种生物质炭的元素组成采用元素分析仪(Flash EA1112,Thermo Finnigan,意大利)测定,表面形貌特征采用扫描电镜(SEM)分析仪(SU-8010,日立公司,日本)分析,表面官能团采用傅里叶红外光谱仪(FTIR)(NICOLET iS10,Thermo Fisher Scientific,美国)测定。利用比表面积及孔隙度仪(TristarⅡ3020,Micromeritica Instument Corporation,美国),根据BET法测定生物质炭比表面积,用干烧法测定灰分质量分数[16]。
-
采用Microsoft Excel 2013进行数据处理,Origin 8.5进行模型拟合及作图。
-
等温吸附模型。Langmuir方程线性式:Ce/Qe=1/(KLQm)+Ce/Qm。其中,Ce表示平衡时溶液中的As(Ⅴ)质量浓度(mg·L−1);Qe表示平衡时单位质量的土壤吸附As(Ⅴ)的量(mg·g−1);Qm为土壤对As(Ⅴ)的饱和吸附量(mg·g−1);KL为吸附速率常数(L·mg−1)。
吸附动力学模型。准一级线性方程(1)、准二级线性方程(2)和颗粒内扩散线性方程(3):
式(1)~式(3)中:t为吸附时间(h);Qt为t时刻时单位质量的土壤吸附As(Ⅴ)的量(mg·g−1);Qe为平衡时单位质量的土壤吸附As(Ⅴ)的量(mg·g−1);K1为准一级反应速率常数(h−1),K2为准二级反应速率常数[mg·(g·h)−1],Kp为颗粒内扩散速率常数(mg·g−1·h0.5);C是由数据代入公式中得出的常数。
1.1. 材料
1.2. 方法
1.3. 土壤和生物质炭性质分析
1.4. 数据分析
1.5. 模型
-
如表1所示:铁改性生物质炭的pH为4.41,较原始生物质炭pH(9.25)明显降低,这是由于经过铁改性处理后的生物质炭表面的铁化合物水解后会产生大量的氢离子(H+),且改性处理会使生物质炭表面的碱性官能团数量减少,从而使生物质炭的pH降低[24]。铁改性生物质炭的灰分含量和电导率均高于原始生物质炭,这一方面是因为铁的负载使铁氧化物增加,另一方面氯离子(Cl−)的大量引入增大了电荷之间的移动性;与原始生物质炭相比,改性后生物质炭的比表面积(74.5 m2·g−1)有所减小,这与王思源等[9]的研究结果相一致,主要是由于改性过程中铁化合物进入生物质炭孔隙内,使部分孔隙被堵塞导致其比表面积减小。此外,改性过后生物质炭的铁质量分数是原始生物质炭的8倍,这也验证了铁材料在生物质炭表面的成功负载。
生物质炭 碳质量
分数/%氢质量
分数/%氮质量
分数/%比表面积/
(m2·g−1)pH 电导率/
(dS·m−1)灰分质量
分数/%阳离子交
换量/(cmol·kg−1)总铁质量
分数/(g·kg−1)原始生物质炭 69.34 2.74 1.11 110.70 9.25 0.37 9.66 21.59 4.72 铁改性生物质炭 59.91 2.24 0.94 74.47 4.41 4.49 15.77 16.70 39.89 Table 1. Properties of the raw and Fe-modified biochars
对比2种生物质炭的扫描电镜图(图1)可以看出,改性前后生物质炭均呈排列均匀的管束结构,说明原始生物质法国梧桐枝条的导管结构经炭化后仍被保存。原始生物质炭表面较为光滑,结构层次更为清晰;在经过FeCl3改性后,铁改性生物质炭的横截面略为粗糙,呈蜂窝状,这可能是改性过程导致生物质炭表面的孔隙被堵塞。这与改性后生物质炭的比表面积降低的结果一致(表1)。
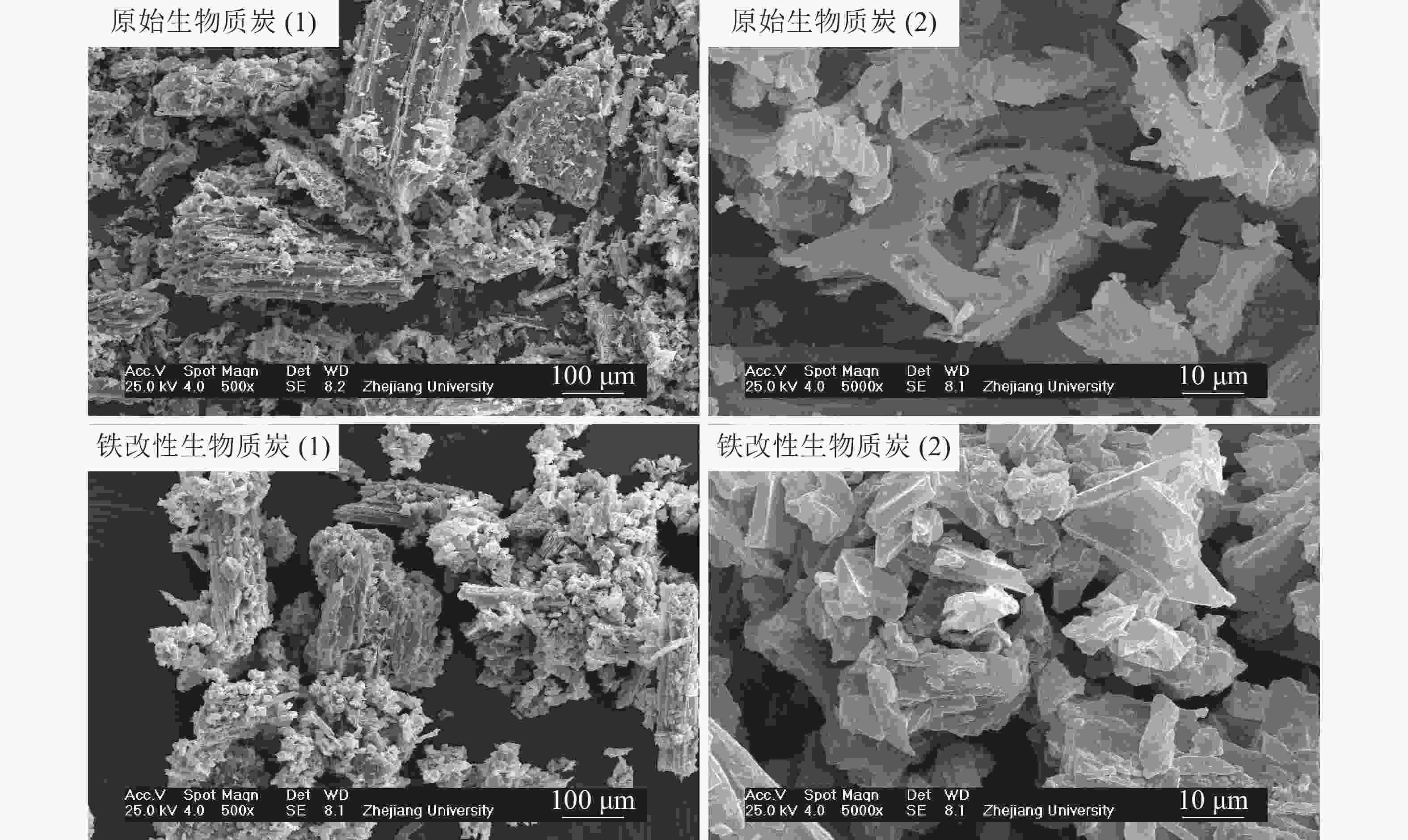
Figure 1. X-ray diffraction scanning electron microscope (SEM) images of raw and iron-modified biochars
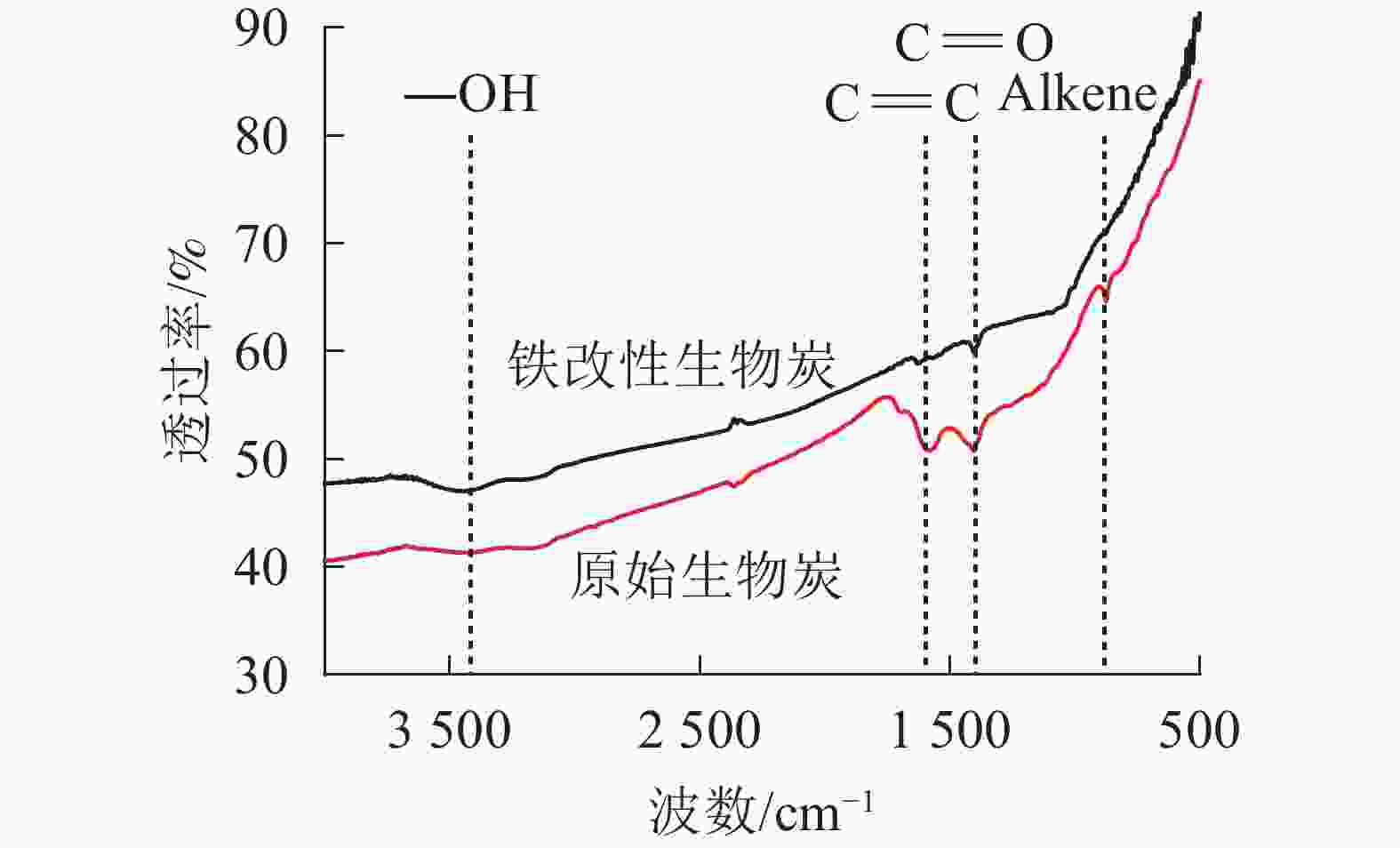
由FTIR图谱(图2)可见:2种生物质炭具有4个大致相同的特征峰,在3 400~3 500 cm−1处的宽峰是由2种生物质炭中羟基(O—H)的伸缩振动形成;1 448~1 576 cm−1处的特征峰主要是由羰基C=O的伸缩振动引起的,又包含共轭的C=C伸缩振动,与SWIATKOWSKI等[25]的研究结果一致。650~1 000 cm−1处表示的是芳香结构取代基C—H面外伸缩振动峰[26]。与原始生物质炭相比,铁改性生物质炭在1 448~1 576 cm−1的峰高强度降低,推断这可能是由生物质炭二次裂解导致部分官能团丢失。杨兴等[4]研究表明:随着热解温度和时间的增加,生物质炭表面的官能团逐渐降低甚至消失。值得注意的是,2种生物质炭在600 cm−1附近的波形图有所差异,有研究表明:该区域为Fe—O基团的弯曲振动[27],这进一步验证了铁材料在生物质炭表面的成功负载。
-
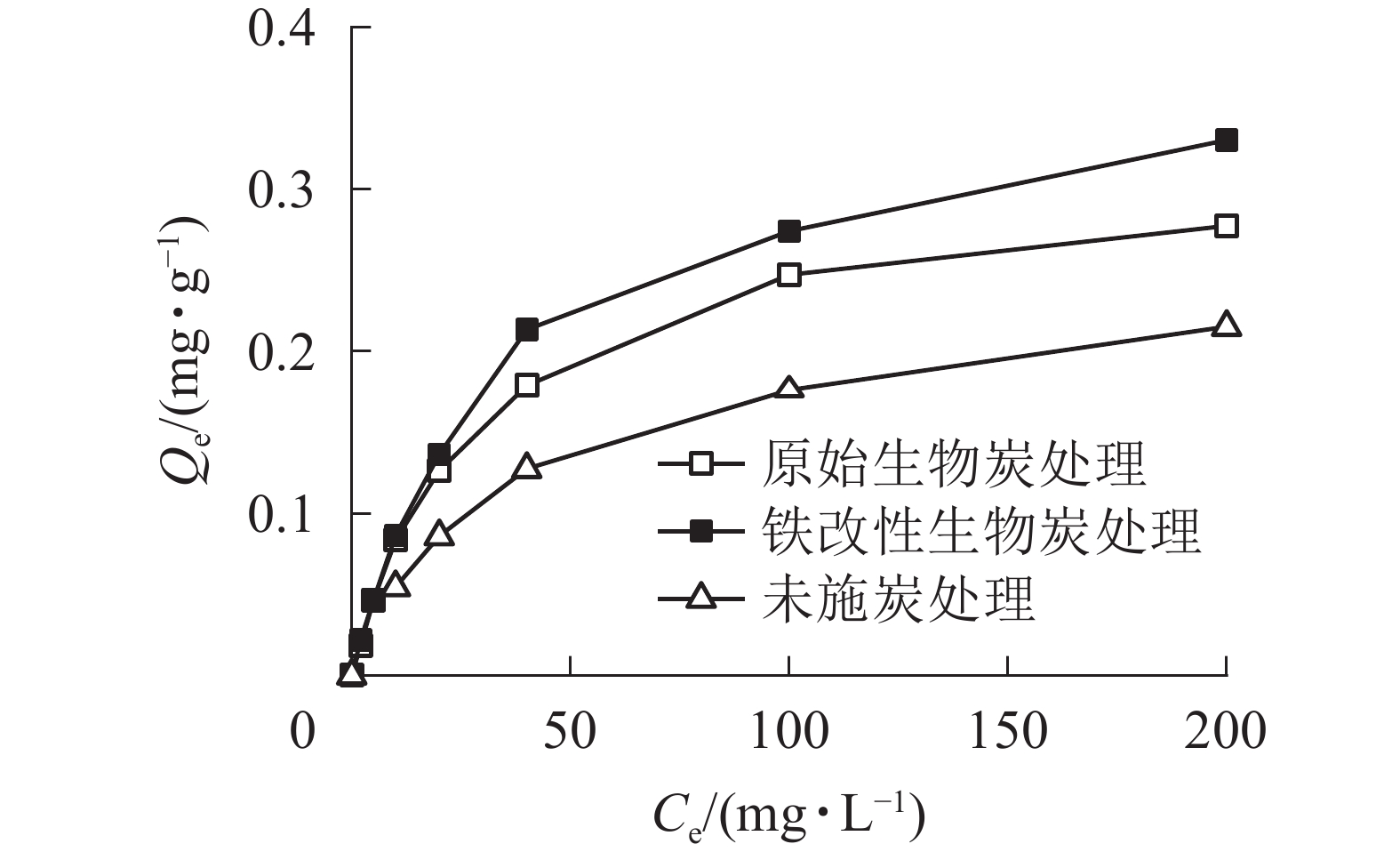
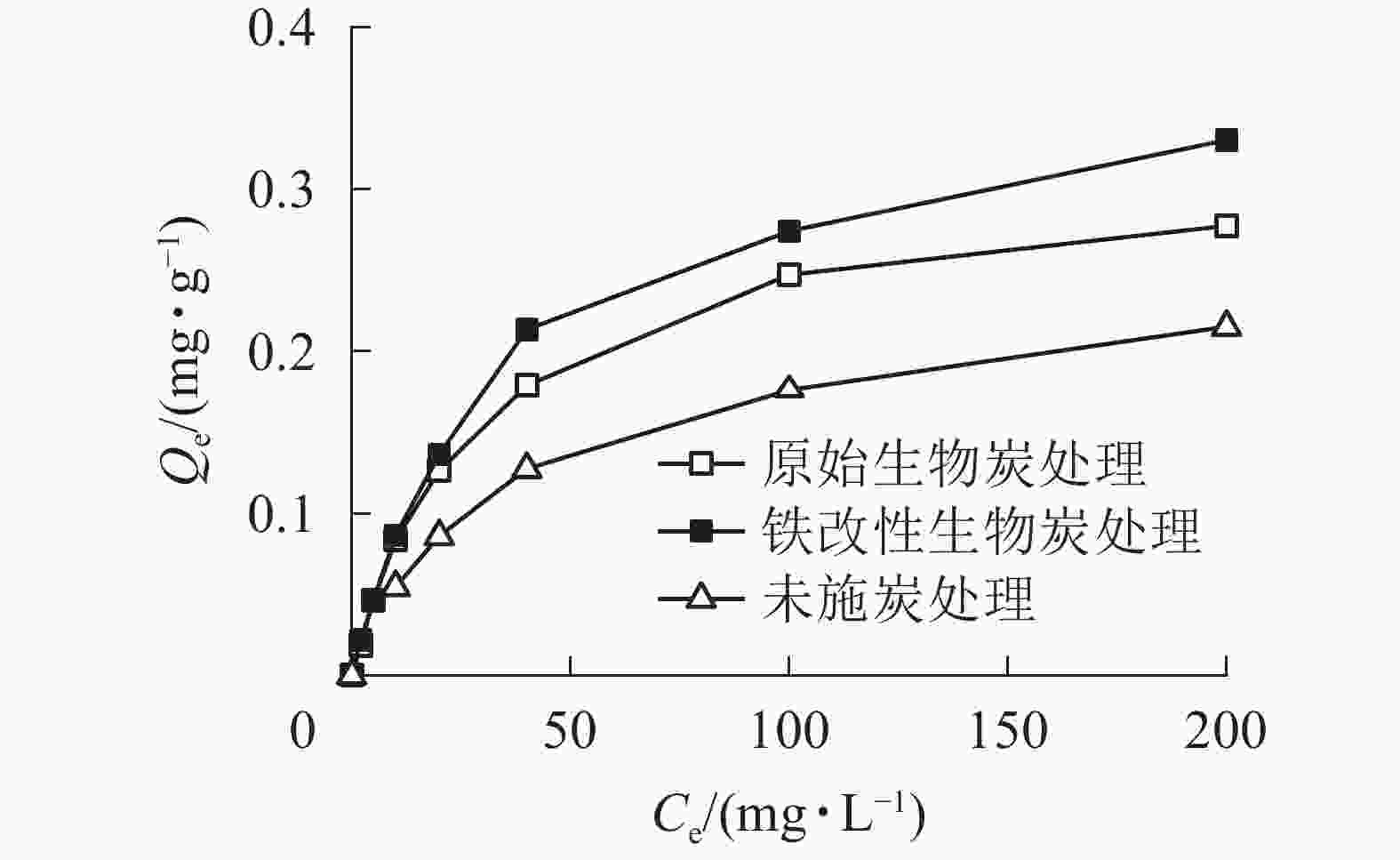
土壤对As的吸附是一个动态平衡的过程,吸附等温线是反映吸附剂与吸附质亲和力强弱的重要依据[28]。由图3可知:溶液中As(Ⅴ)的初始质量浓度与平衡吸附量之间关系密切,2种施炭土壤对As(Ⅴ)的平衡吸附量随As(Ⅴ)初始质量浓度的升高而逐渐增大,增加的速度遵循先快后慢的规律,最后趋于平衡。2种施炭土壤的吸附过程基本一致,当As(Ⅴ)溶液初始质量浓度小于25 mg·L−1时,2种施炭土壤对As(Ⅴ)的平衡吸附量也基本一致;但当As(Ⅴ)初始质量浓度大于25 mg·L−1后,铁改性生物质炭处理对As(Ⅴ)的平衡吸附量逐渐大于原始生物质炭,As(Ⅴ)初始质量浓度为200 mg·L1时其平衡吸附量比原始生物质炭高19%。由于在一定条件下土壤对As(Ⅴ)的吸附点位是一定的,随着As(Ⅴ)质量浓度的增加,有限的吸附点位被占据,逐渐减少的吸附位点使土壤对As(Ⅴ)的吸附趋于缓慢[29]。
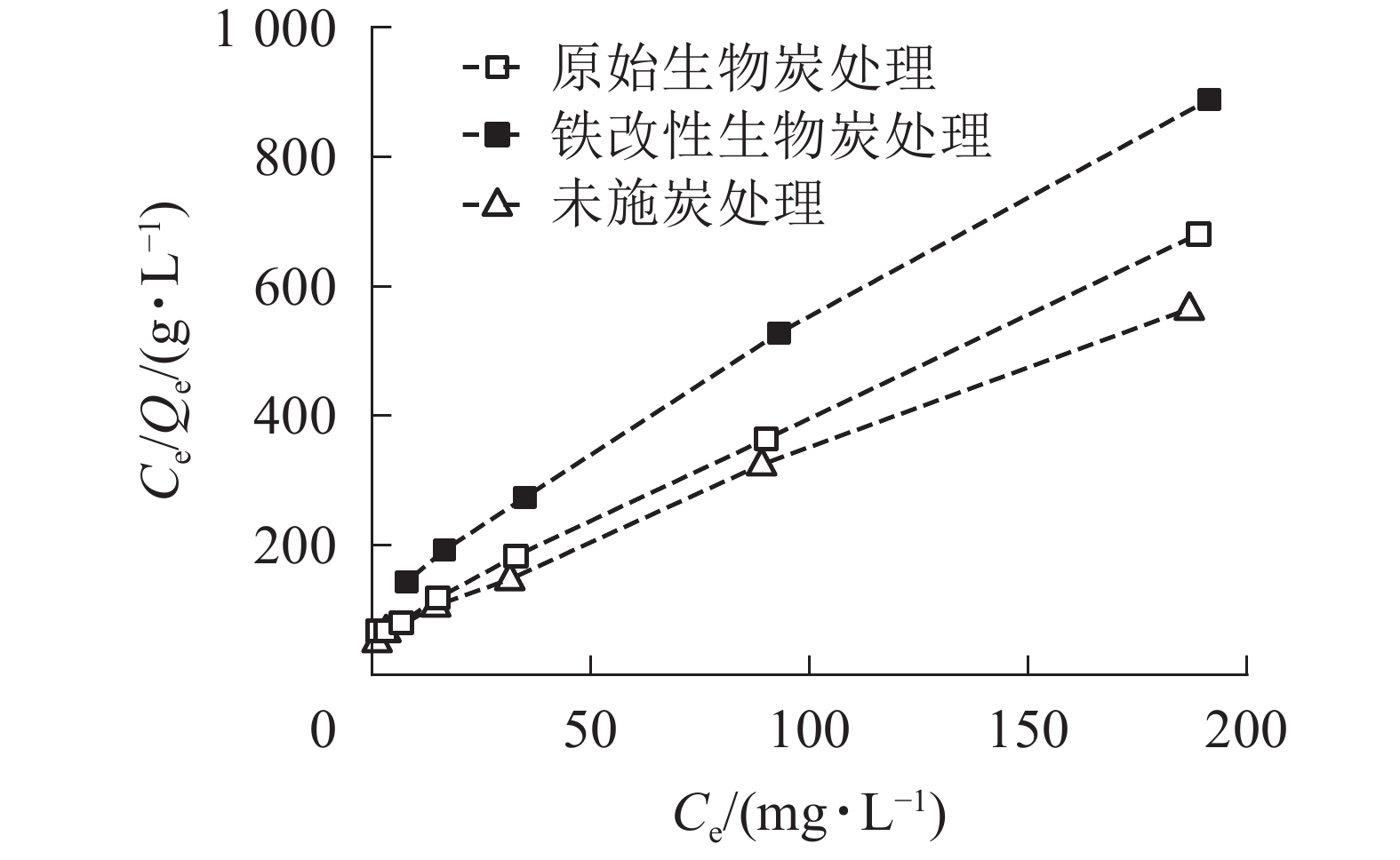
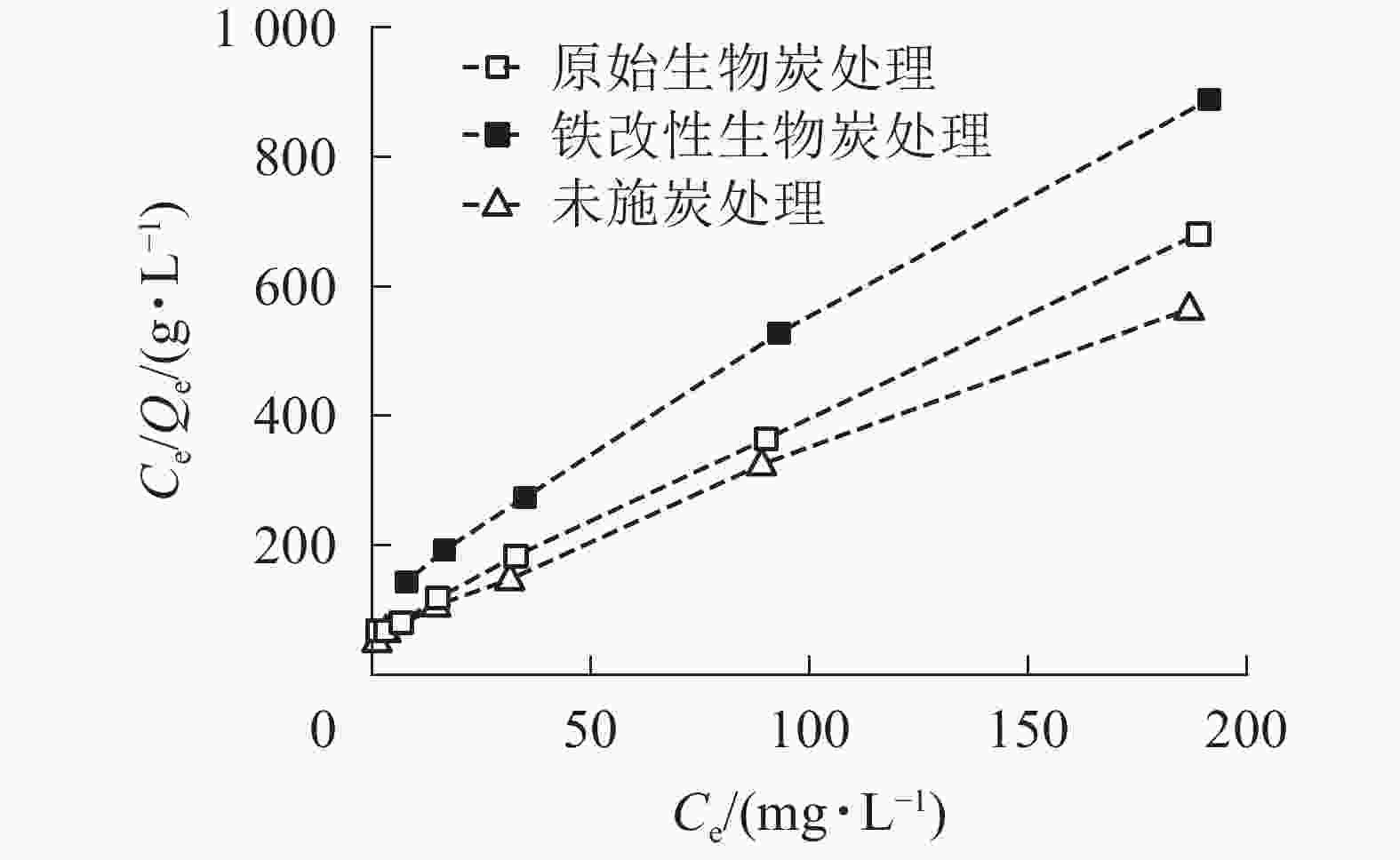
本研究采用Langmuir等温吸附模型对数据进行拟合。从拟合结果(表2,图4)可知:Langmuir方程的相关系数R2为0.997~0.999,能较好地对数据进行拟合,这表明施炭土壤对As(Ⅴ)的整个吸附过程以单分子层吸附为主[30]。Langmuir等温吸附模型中对照土壤、原始生物质炭和铁改性生物质炭的最大吸附量分别为0.25、0.31和0.36 mg·g−1。铁改性生物质炭对As(Ⅴ)的吸附量显著高于对照土壤和原始生物质炭处理,其最大吸附量Qm较未施炭对照土壤和原始生物质炭处理分别提高了44.0%和16.1%。该结果与等温吸附试验结果一致。
处理 Qm/(mg·g−1) KL/(L·mg−1) R2 未施炭处理 0.25 0.032 0.997 原始生物质炭处理 0.31 0.051 0.999 铁改性生物质炭处理 0.36 0.046 0.997 Table 2. Parameters of Langmuir isotherms for the adsorption of As(Ⅴ) on the control and biochar-treated soils
-
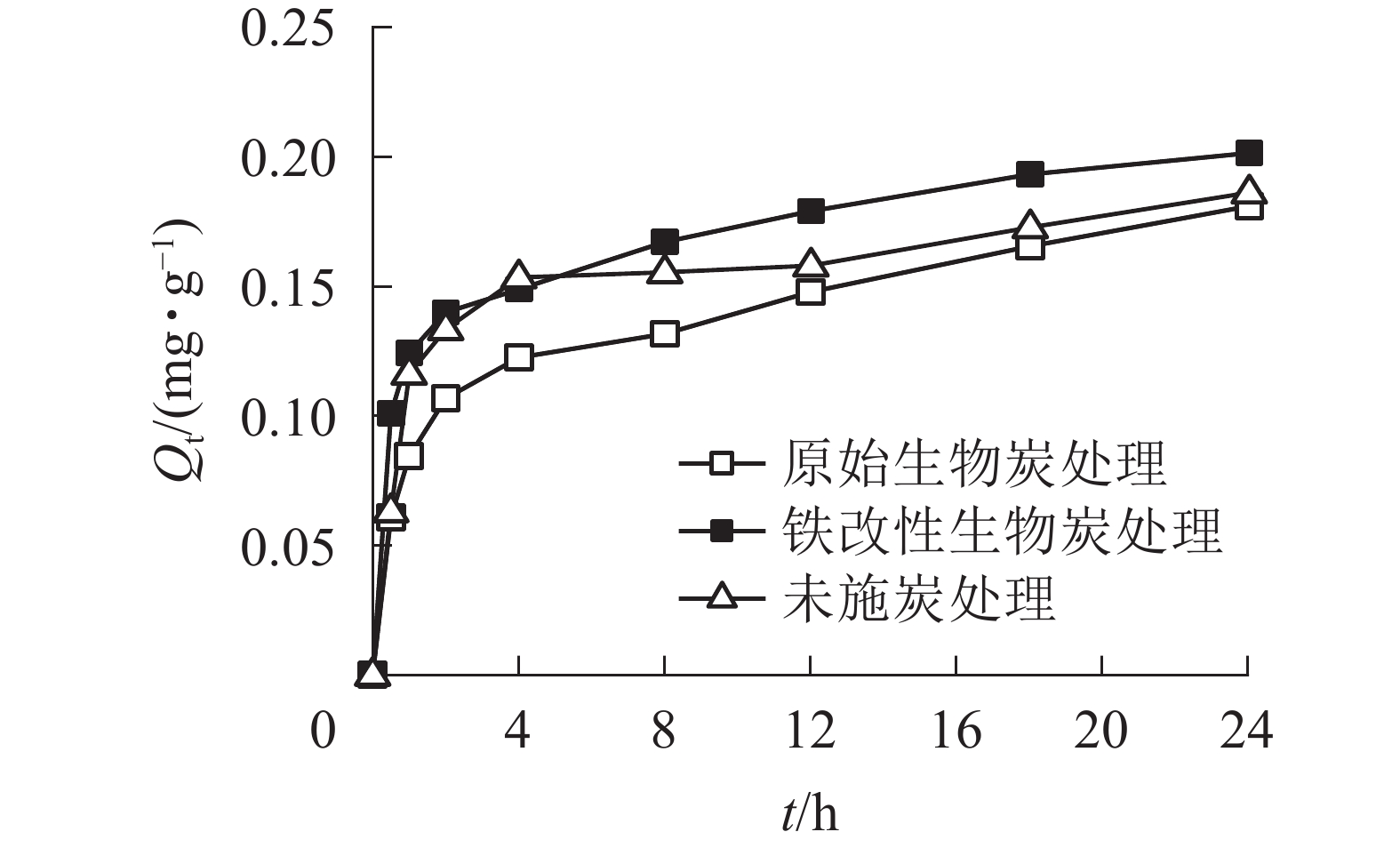
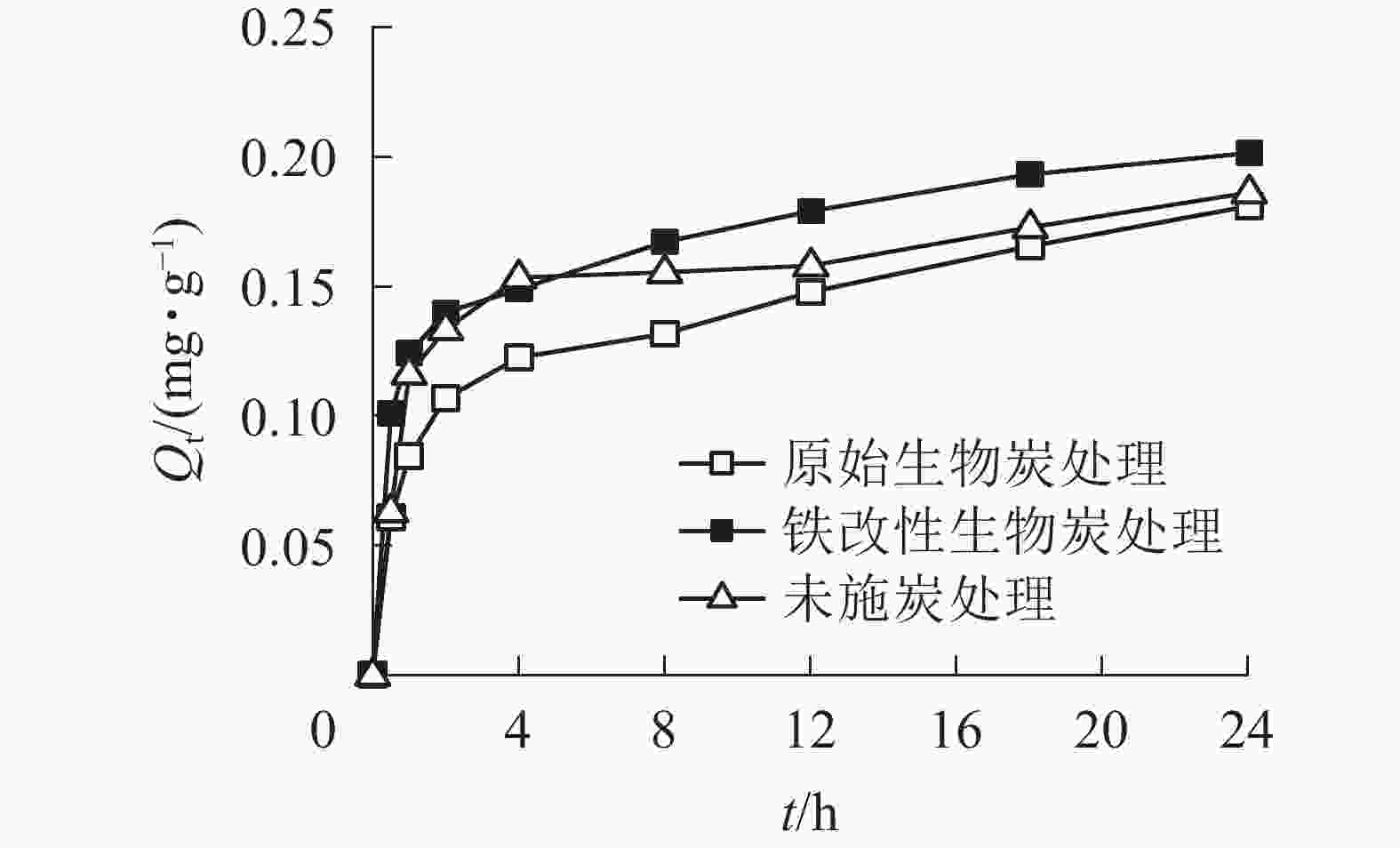
如图5所示:2种施炭处理的土壤对As(Ⅴ)的吸附量随着吸附时间的增加而增大。整个吸附过程可分为2个阶段:第1阶段是0~4 h的快速吸附阶段,在4 h内原始生物质炭和铁改性生物质炭的吸附量已达到饱和吸附量的67.7%和73.9%;第2阶段为4~24 h的慢速吸附阶段,吸附量增长速率减慢至饱和。未施炭处理、原始生物质炭和铁改性生物质炭处理的平衡吸附量分别为0.186、0.181和0.201 mg·g−1,铁改性生物质炭处理的土壤的平衡吸附量比未施炭处理高8.1%、比原始生物质炭处理高11.0%,而原始生物质炭处理的土壤的平衡吸附量比未施炭处理低2.8%。在4~24 h的慢速吸附过程中,随着吸附剂的吸附位点逐渐被占据后达到饱和,As(Ⅴ)从外部进入内部位点的速度相对较慢,吸附量增加也相对缓慢。此过程占主导的吸附方式是以表面吸附为主的化学吸附过程[31]。
为了更好地描述施炭土壤对As(Ⅴ)的吸附动力学特性,本研究采用准一级动力学方程、准二级动力学方程和颗粒内扩散方程对数据进行拟合(图6,表3)。原始生物质炭和铁改性生物质炭处理的准二级动力学方程的相关系数分别为0.928和0.974,大于准一级线性方程的拟合度,且通过准二级动力学方程计算所得的平衡吸附量与实际吸附量更为接近,因此2种施炭土壤的整个吸附过程更符合准二级动力学方程,表明整个吸附过程以化学吸附为主[32]。准二级动力学方程可以描述化学吸附的所有过程,包括外部液膜扩散、表面吸附、颗粒内扩散等,能更好地描述快速与慢速相互叠加的吸附过程[33-34]。在准二级动力学方程中,未施炭处理、原始生物质炭和铁改性生物质炭处理的平衡吸附量分别为0.186、0.181和0.201 mg·g−1,铁改性生物质炭的平衡吸附量高于未施炭处理和原始生物质炭处理,且铁改性生物质炭处理的K2大于原始生物质炭处理,说明施用铁改性生物质炭土壤的吸附速率高于施用原始生物质炭土壤。为了进一步探究施炭土壤对As(Ⅴ)的具体吸附过程,本研究结合颗粒内扩散方程进行分析。由图6可知:施炭土壤对As(Ⅴ)的吸附曲线是一条不通过原点的直线,说明颗粒内扩散不是唯一扩散方式,而是由外部液膜扩散和颗粒内扩散共同组成[35]。本研究中土壤对As(Ⅴ)的吸附过程可分为2个阶段:第1阶段为外部液膜扩散,即溶液中的As(Ⅴ)被吸附到吸附剂表面的过程;第2阶段为颗粒内扩散,即As(Ⅴ)在施炭土壤表面间由外部向内部层间扩散。对比实际吸附过程和曲线拟合结果可得,施用铁改性生物质炭的土壤对As(Ⅴ)的吸附速率和平衡吸附量均大于原始生物质炭处理。
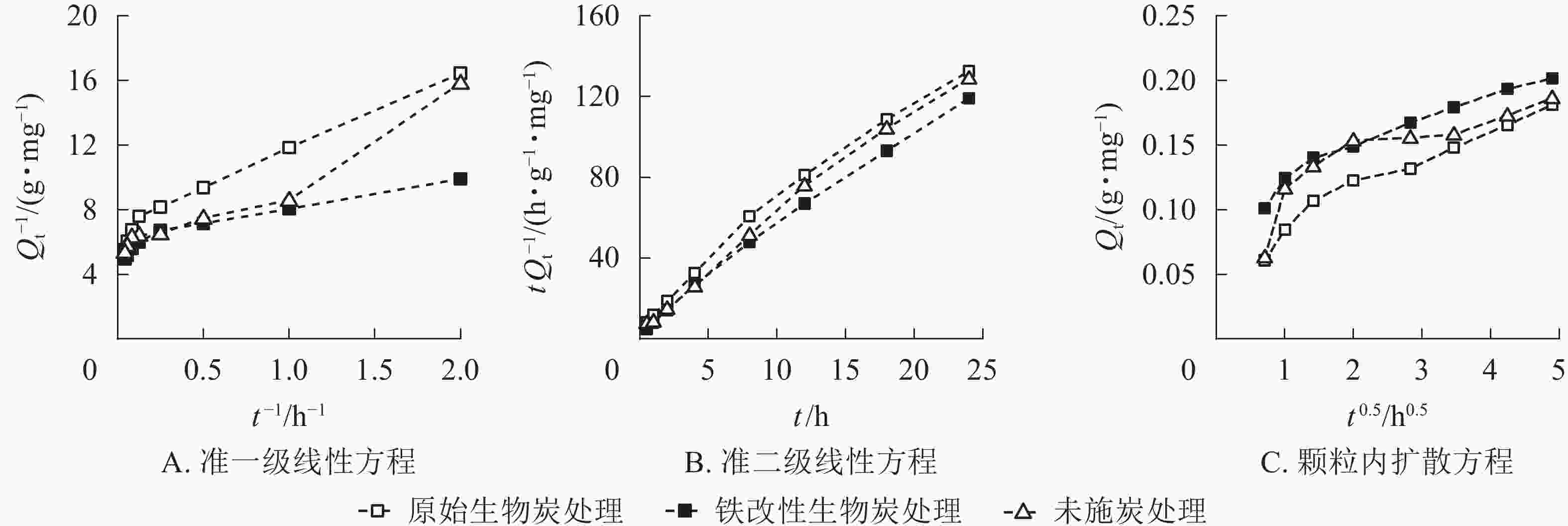
Figure 6. Sorption kinetic curves of the pseudo-first-order kinetics (A), the pseudo-second-order kinetics (B) and internal diffusional models (C) for the adsorption of As(Ⅴ) on the control and biochar-treated soils
处理 准一级动力学方程 准二级动力学方程 颗粒内扩散方程 K1/h−1 R2 Qe/
(mg·g−1)K2/
(mg·g−1·h−1)R2 Qe/
(mg·g−1)Kp1/
(mg·g−1·h−1)Kp2/
(mg·g−1·h−1)R12 R22 未施炭处理 0.909 0.952 0.187 4.865 0.994 0.185 0.063 2 0.015 4 0.834 0.946 原始生物质炭处理 0.827 0.974 0.580 2.926 0.988 0.185 0.035 2 0.018 3 0.882 0.991 铁改性生物质炭处理 0.425 0.928 0.182 4.371 0.996 0.206 0.046 6 0.020 8 0.944 0.983 Table 3. Parameters of kinetic models for the adsorption of As(Ⅴ) on the control and biochar-treated soils
2.1. 改性前后生物质炭的基本理化性质
2.2. 施用生物质炭后土壤对As(Ⅴ)的等温吸附研究
2.3. 2种生物质炭加入土壤对As(Ⅴ)的吸附动力学研究
-
本研究以园林废弃物法国梧桐修剪枝作为原料,制备了原始生物质炭和铁改性生物质炭。铁改性生物质炭较原始生物质炭的pH、比表面积及官能团数量降低、但灰分质量分数和电导率有所增加。Langmuir等温吸附方程和准二级动力学方程能更好地描述施炭土壤对As(Ⅴ)的吸附过程,最大吸附量分别为0.31和0.36 mg·g−1。吸附过程以化学吸附为主,又包含液膜扩散和颗粒内扩散共同作用。铁改性生物质炭施入土壤后明显提高了土壤对As(Ⅴ)的吸附能力,吸附速率和吸附量均高于未施炭土壤和原始生物质炭处理。
生物质炭负载铁后对As的修复可能存在以下几种机制:①经铁改性后生物质炭pH显著降低,加入土壤中可降低土壤pH,从而使土壤胶体所带正电荷增加[36],而As在土壤中大多是以AsO43−或AsO33−等阴离子形态存在,因此可通过静电吸附使As固定吸附在土壤胶体表面[37];②铁改性生物质炭能促进As在铁氧化物表面形成稳定的单齿或双齿配位体,进而降低As的移动性[38];③将铁改性生物质炭施入土壤后,其游离铁离子易与As形成较稳定的Fe−As共沉淀物[39]。基于此,施用铁改性生物质炭的土壤比施用未改性生物质炭的土壤对As(Ⅴ)的吸附能力更强。










 DownLoad:
DownLoad: