-
黄曲霉毒素(aflatoxins, AFs)主要是由黄曲霉Aspergillus flavus和寄生曲霉Aspergillus parasiticus产生的有毒次级代谢物,可通过污染食品和饲料进入食物链,严重威胁动物和人类健康[1]。目前,国内外已发现超20种AFs,其中黄曲霉毒素B1(AFB1)毒性最强,危害最大,已被国际癌症研究机构(IARC)列为Ⅰ类致癌物[2-4]。AFB1具有强烈的“致突变、致癌和致畸作用”和免疫毒性,过量摄入可破坏人和动物的肝脏组织,引发急性中毒,长期摄入则会引发各组织器官癌变[5-6]。建立快速、高灵敏的AFB1检测方法对于保障食品安全具有重要意义。在AFB1检测手段中,仪器法如高效液相色谱法(HPLC)、气相色谱法(GC)和液相色谱-串联质谱法(LC-MS/MS)等虽灵敏度高,准确性和重现性较好,但设备耗材昂贵且操作繁琐,难以用于样本的初筛和在基层使用[7-10];相比仪器分析法,基于抗原抗体反应的免疫分析法因操作简单、灵敏度高且特异性好,在真菌毒素检测领域应用较广,特别是免疫层析法,省时高效且无需借助复杂仪器,尤其适合大量样本的现场筛查[11-16]。本研究基于免疫层析技术原理,采用金颗粒标记AFB1单克隆抗体,在竞争反应模式下,优化金颗粒尺寸、层析体系各组成材料及相关缓冲液配方,最终建立AFB1高灵敏定性定量免疫层析检测法,通过肉眼直接对检测结果定性判定,或借助便携式信号读取设备实现定量分析,以满足对AFB1污染情况快速筛查的检测需求。
-
牛血清白蛋白(BSA)、卵白蛋白(OVA)、N-羟基琥珀酰亚胺(NHS)、羧甲基羟胺半盐酸盐(CMO)、N,N-二环已基碳二亚胺(DCC)、二甲基亚砜(DMSO)、各真菌毒素标准品购自Sigma公司;AFB1单克隆抗体(Anti-AFB1)、硝酸纤维素膜和玻璃纤维等免疫层析耗材购自奥唯生物;黄曲霉毒素B1商品化检测试剂盒购自无锡景麒生物;其他试剂购自上海国药;谷物样本由浙江省检验检疫科学技术研究院提供。
-
选取不同载体蛋白(BSA和OVA)制备AFB1完全抗原。步骤:AFB1标准品(2 mg)溶解于甲醇-吡啶溶液(体积比1∶1),加入5 mg CMO震荡至完全溶解,70 ℃下搅拌活化2 h;活化产物自然干燥后,加入1 mL蒸馏水并使用氢氧化钠溶液(1 mol·L−1)调pH至8.0,为去除体系中未反应的AFB1,使用5 mL苯溶液抽提3次,pH调至3.0 (0.2 mol·L−1盐酸),产物经5 mL乙酸乙酯抽提3次后干燥得到AFB1肟化物AFB1O;所得AFB1O溶解于2 mL二甲基甲酰胺(DMF),分别加入DCC (8.9 mg)和NHS (5.0 mg)室温搅拌活化4 h;BSA (14.0 mg)或OVA (9.3 mg)溶解于1 mL碳酸氢钠溶液(0.1 mol·L−1,pH 9.5),将上一步所得的AFB1O活化产物缓慢滴加至载体蛋白溶液,室温混匀反应2 h;反应结束后在磷酸盐缓冲液(0.01 mol·L−1,pH 7.4)中4 ℃透析72 h,每12 h换液,产物即为AFB1完全抗原,经酶联免疫检测法(ELISA)鉴定后保存备用。
-
采用柠檬酸钠还原法分别制备不同粒径的金颗粒(20和40 nm)用于单克隆抗体的标记[17]。具体步骤:250 mL三角烧瓶(在浓硫酸-重铬酸钾溶液中浸泡并使用去离子水冲洗干净)置于磁力搅拌加热器,加入100 mL质量分数为0.01%氯金酸溶液加热至沸腾,迅速加入0.750或0.375 mL质量分数为2%的柠檬酸钠溶液分别制备20和40 nm粒径的金颗粒,搅拌加热至混合液颜色至酒红色,调低功率继续加热5 min,室温自然冷却后即得到金颗粒溶液,采用目测法和透射电镜扫描鉴定后备用。
-
金颗粒标记抗体最佳pH和抗体标记最佳结合质量浓度参考文献[18]。金颗粒标记抗体步骤:使用10 mmol·L−1Tris-HCL溶液(pH 7.4)稀释单克隆抗体Anti-AFB1至最佳结合质量浓度,金颗粒溶液经0.2 mol·L−1碳酸钾调节至抗体标记最佳pH;取50 mL已调节pH的金颗粒溶液,边搅拌边加入待标记抗体,室温混匀30 min;反应结束后加入BSA溶液(终质量分数为1%),混匀30 min后4 ℃ 2 000 g离心30 min,弃沉淀;上清8 000 g 离心30 min,将所得沉淀重悬于10 mL 2 mmol·L−1含质量分数为1% BSA的硼酸盐缓冲液(pH 7.4),8 000 g离心20 min去除未结合的抗体,重复2次;所得沉淀溶解于5 mL硼酸盐缓冲液,4 ℃保存备用。
-
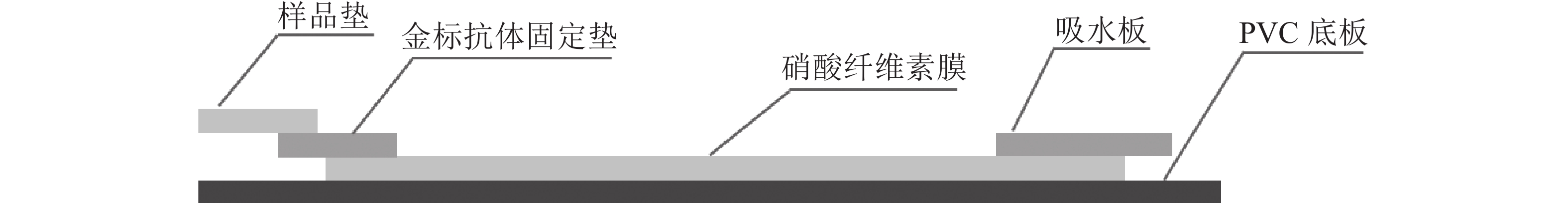
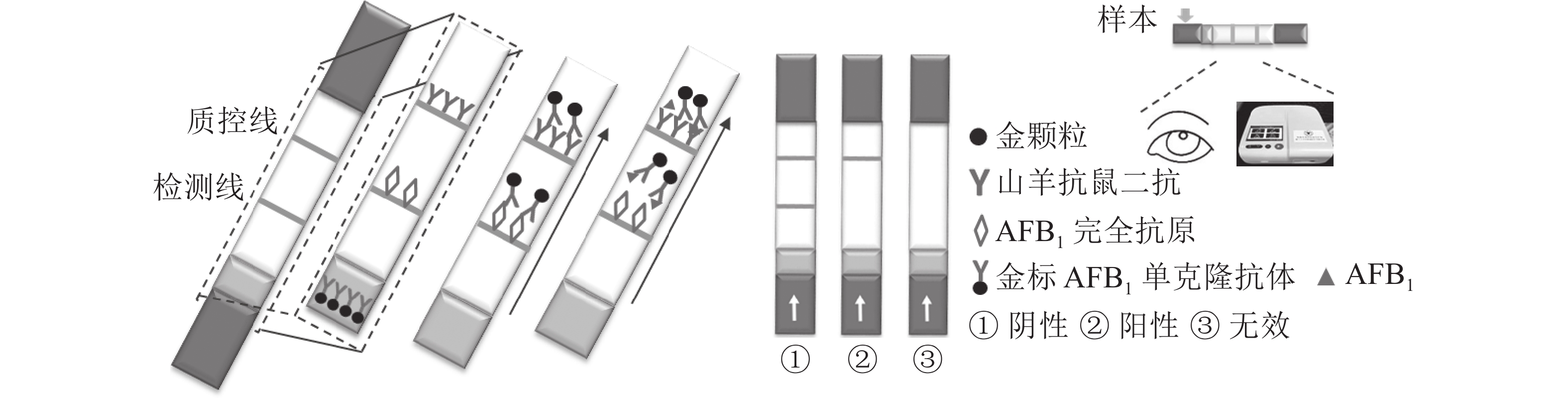
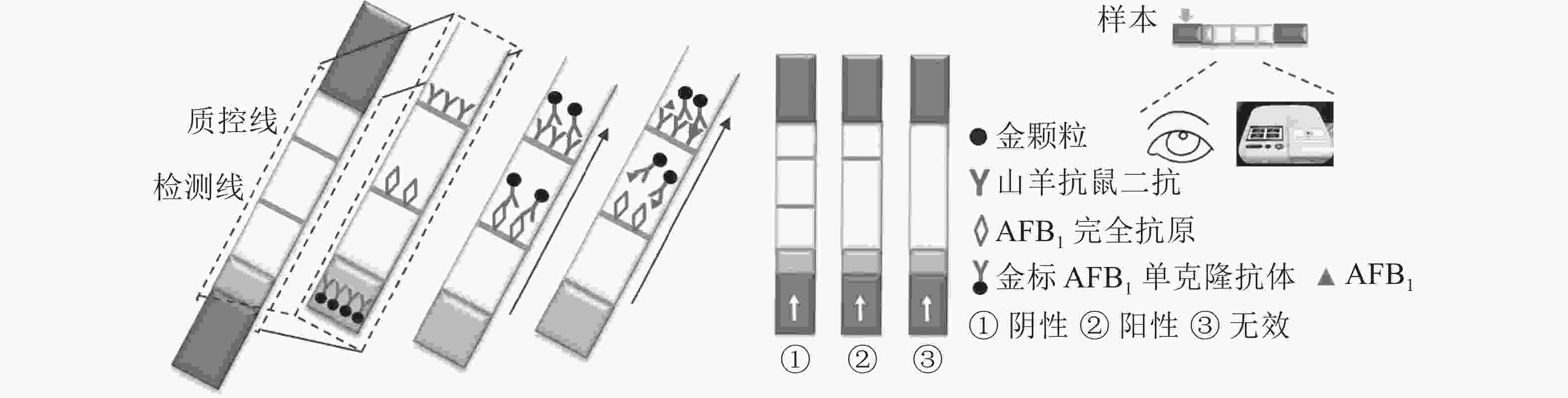
定性定量免疫层析检测法如图1所示。检测线包被AFB1完全抗原,质控线包被山羊抗鼠二抗,金颗粒标记的Anti-AFB1固定于金标垫。滴加待检样品,经层析作用后,可通过肉眼观察检测线与质控线颜色差异进行定性判定或经便携式信号读取仪检测线信号值进行定量分析。
-
免疫层析法检测效果受多种参数影响,如标记抗体所用金颗粒粒径、检测线包被抗原类型、层析各组分材料品种(包括硝酸纤维素膜、金标抗体固定垫和样品垫)和各组分材料前处理液的类型和浓度[19-21]。优化策略:采用不同粒径金颗粒标记单克隆抗体,评价稳定性和敏感性,选取最优;对不同硝酸纤维素膜(Millipore 135、Millipore 180、Pall 170和Sartorius CN 140)、金标抗体固定垫(Ahlstrom 8964、Ahlstrom 6613、国产GF06和国产GF08)和样品垫(国产SB08和SB06)层析效果进行比较;以金标抗体稳定性和检测效果为标准,优化金标抗体保存液、抗原包被液、金标抗体固定垫和样品垫前处理液的最佳配方与浓度;确定样本萃取液稀释倍数,在消除基质影响的同时,获得最佳检测灵敏度。上述各参数优化均参考文献[22]进行。
-
优化处理后的样品垫、金标固定垫、硝酸纤维素膜和吸水板按图2所示粘贴于PVC底板,相邻部分依次重叠,压实并切割成条(宽0.5 cm)。取100 μL梯度质量浓度的AFB1标准液滴至加样孔,15 min后判定检测结果。
-
样本萃取:取待检样本5 g置于250 mL三角烧瓶,分别加入1 g氯化钠和25 mL甲醇-水溶液(体积比7∶3),剧烈震荡15 min,4 000 g离心5 min后过滤,所得萃取液经超纯水稀释后待检。样本加标:阴性样本烘干后研磨过筛,加入AFB1标准品溶液,充分混匀后,室温过夜放置后待检。
-
采用37 ℃加速试验判定稳定性[18]。组装密封好的试纸条置于37 ℃恒温箱,不同天数(7、15、30 d)后取出,对其检测灵敏度进行评价,预测常温储存保存期。
-
采用免疫层析检测法、商品化检测试剂盒和LC-MS/MS平行检测天然AFB1阳性样本,并对检测结果的一致性进行分析。
-
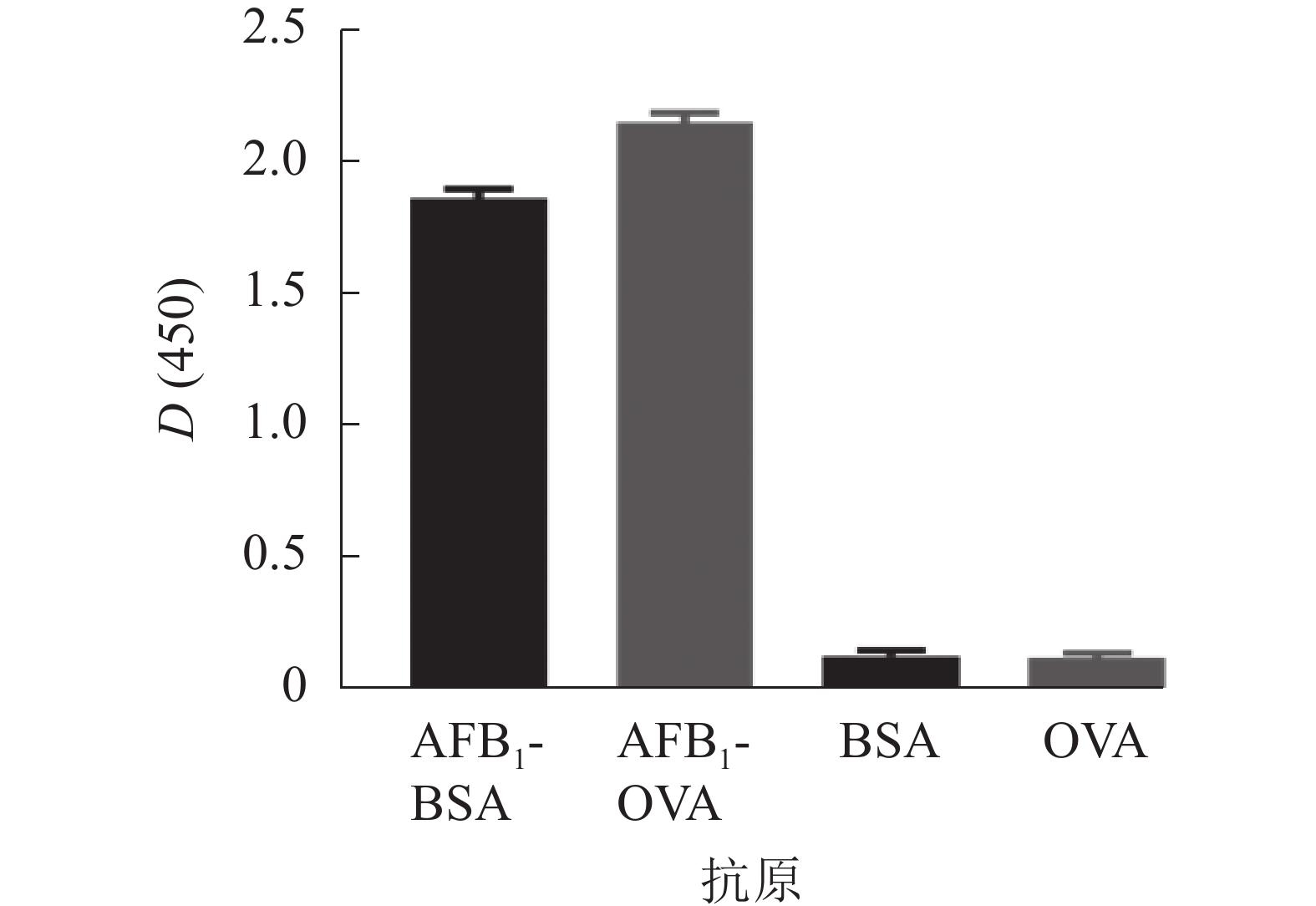
采用AFB1单克隆抗体对AFB1-BSA/OVA进行ELISA鉴定,结果如图3所示,完全抗原组D(450)与对照比值(BSA/OVA)远大于2.1,表明制备成功,可用于免疫学方法的建立。
-
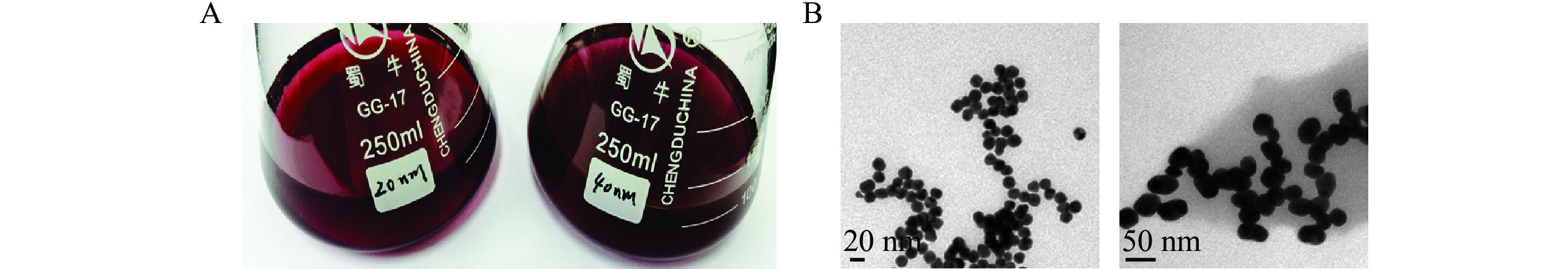
制备的金颗粒溶液颜色澄清鲜艳,40 nm金颗粒溶液颜色较20 nm深,无颗粒沉淀(图4A),透射电镜扫描结果显示颗粒粒径与预期相符,尺寸均匀(图4B),可用于后续抗体的标记。
-
40和20 nm金颗粒标记物层析效果相当,但4 ℃储存时前者性质更稳定,可保存4周,因此综合考虑灵敏度和稳定性,后续试验将采用40 nm金颗粒进行单克隆抗体的标记。
-
包被AFB1-BSA时,检测线显色清晰且灵敏度更好,后续试验将采用AFB1-BSA作为检测线包被抗原。
-
层析组分材料会影响层析灵敏度、时间和稳定性。对各组分材料进行筛选,硝酸纤维素膜的比较结果显示:Sartorius CN 140相较于其他,流动性更佳且检测线不易弥散,层析15 min后即可判定结果,背景值低,为最优,封闭液为含质量分数为0.5%吐温20 (Tween-20)、1%聚乙二醇2000 (PEG 2000)、2%BSA和0.01%叠氮钠(NaN3)的10 mmol·L−1磷酸盐缓冲液(pH 7.4,PBS);金标抗体固定垫的比较结果显示:Ahlstrom 8964上固定的金标抗体可在15 min内释放完全且无聚沉,为最优,处理液为含质量分数为4%蔗糖、1%BSA和0.25%表面活性剂TritonX-100的50 mmol·L−1硼酸盐缓冲液(pH 7.4,BB);样品垫的比较结果显示:国产SB08对含甲醇、纤维素和蛋白质的样本承载能力和缓冲能力更强,为最优,前处理液种类与金标固定垫相同。
-
对含不同质量浓度海藻糖、NaN3和OVA的10 mmol·L−1硼酸盐缓冲液(pH 7.4,BB)在4 ℃条件下对金标抗体的储存和稀释效果进行比较,结果显示:在质量分数为10%海藻糖、1% BSA的条件下,金标抗体复溶效果较好且可稳定保存30 d,最终确定金标抗体存储稀释液为含质量分数为10%海藻糖、1%BSA和0.05%NaN3的10 mmol·L−1的硼酸盐缓冲液(pH 7.4,BB)。抗原包被液优化结果显示:含体积分数为3%甲醇的10 mol·L−1硼酸盐缓冲液(pH 9.0,BB)可使检测线颜色更加均匀和清晰。
-
为获得最佳的检测效果,对完全抗原包被质量浓度和金标抗体的使用质量浓度进行优化,最终确定AFB1-BSA的包被质量浓度为0.4 g·L−1,10倍稀释后的金标AFB1单克隆抗体喷涂量为20 μL·cm−2。
-
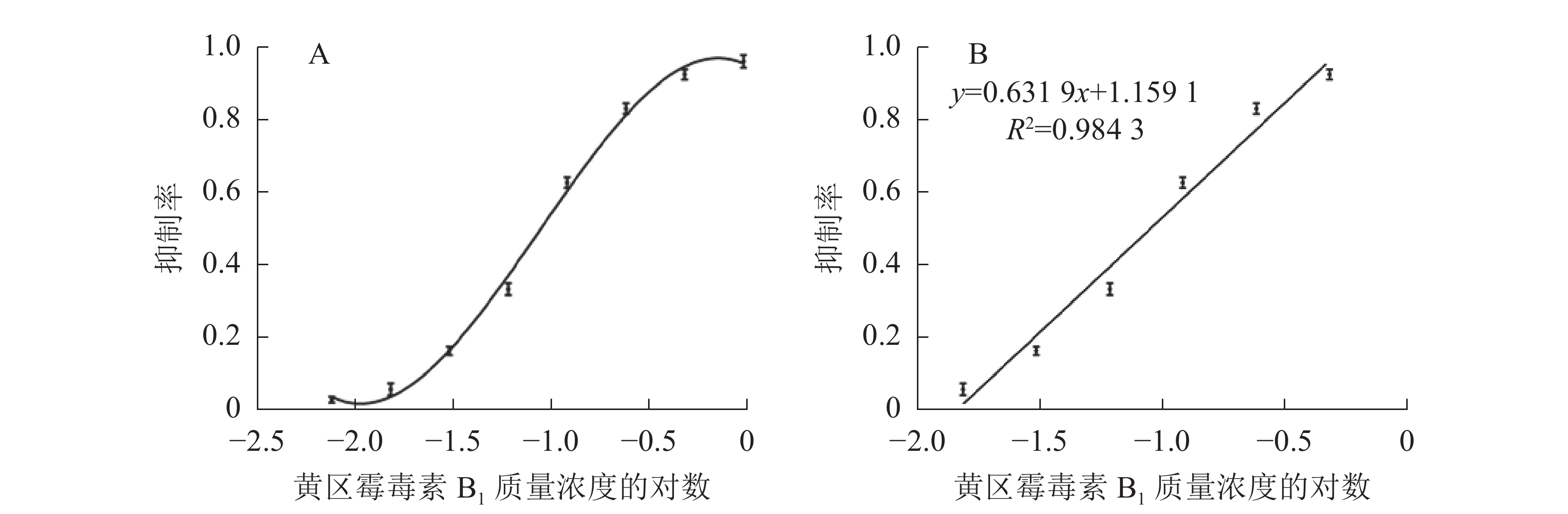
如图5所示:与对照相比,随着AFB1质量浓度的升高,检测线逐渐变浅直至消失,肉眼条件下,使检测线质量浓度发生明显变化的最低标准品质量浓度即为检测限,因此,本免疫层析法的检测限为0.10 μg·L−1。配制系列梯度质量浓度的AFB1标准品溶液进行检测,层析结束后使用便携式信号读取仪分析检测线信号强度。以标准品质量浓度(x)为横坐标,检测线信号强度抑制率(y)为纵坐标,绘制标准抑制曲线,进行线性回归分析(图6),线性方程为y=0.631 9x+1.159 1,R2=0.984 3,定量区间为0.03~0.27 μg·L−1,检测下限为0.02 μg·L−1。
-
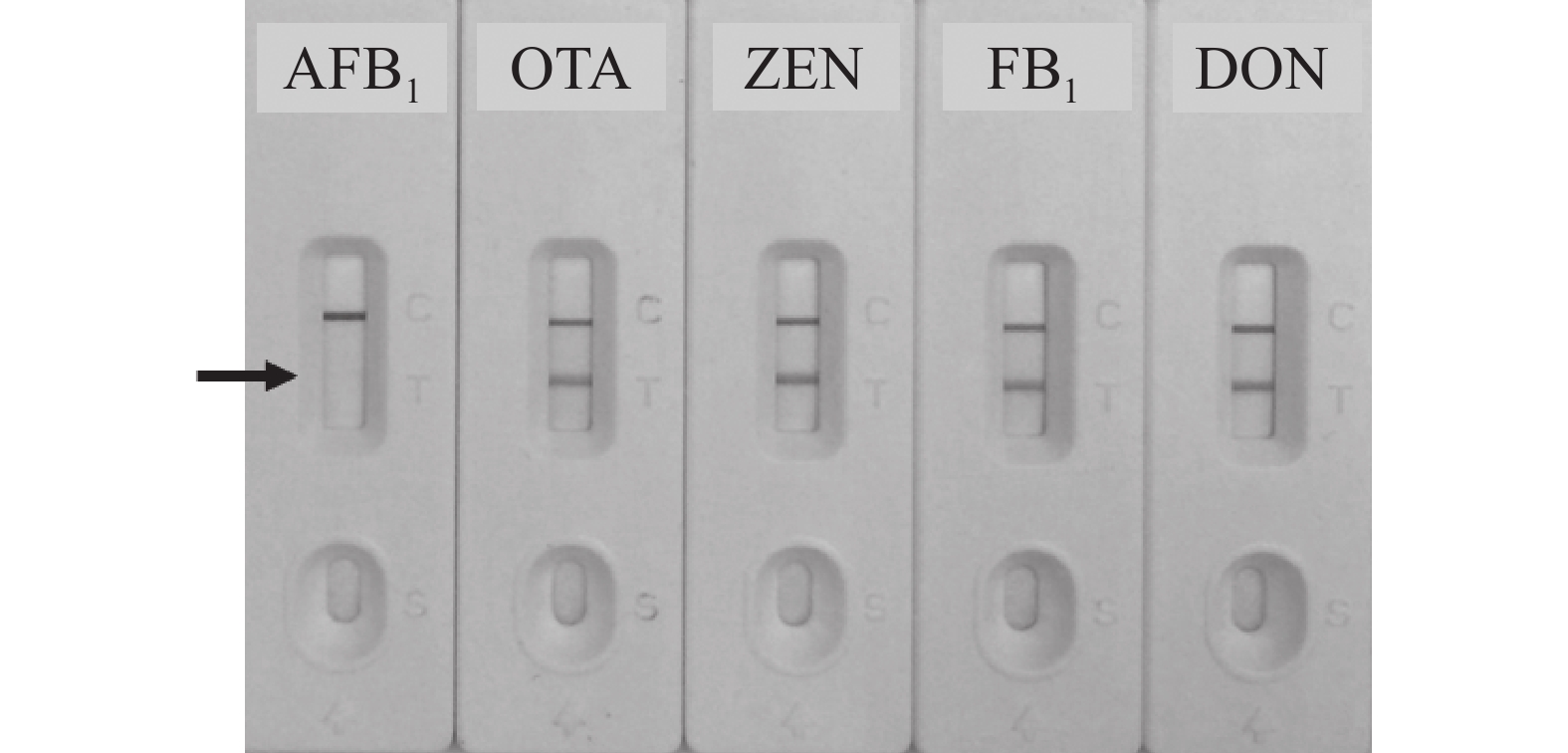
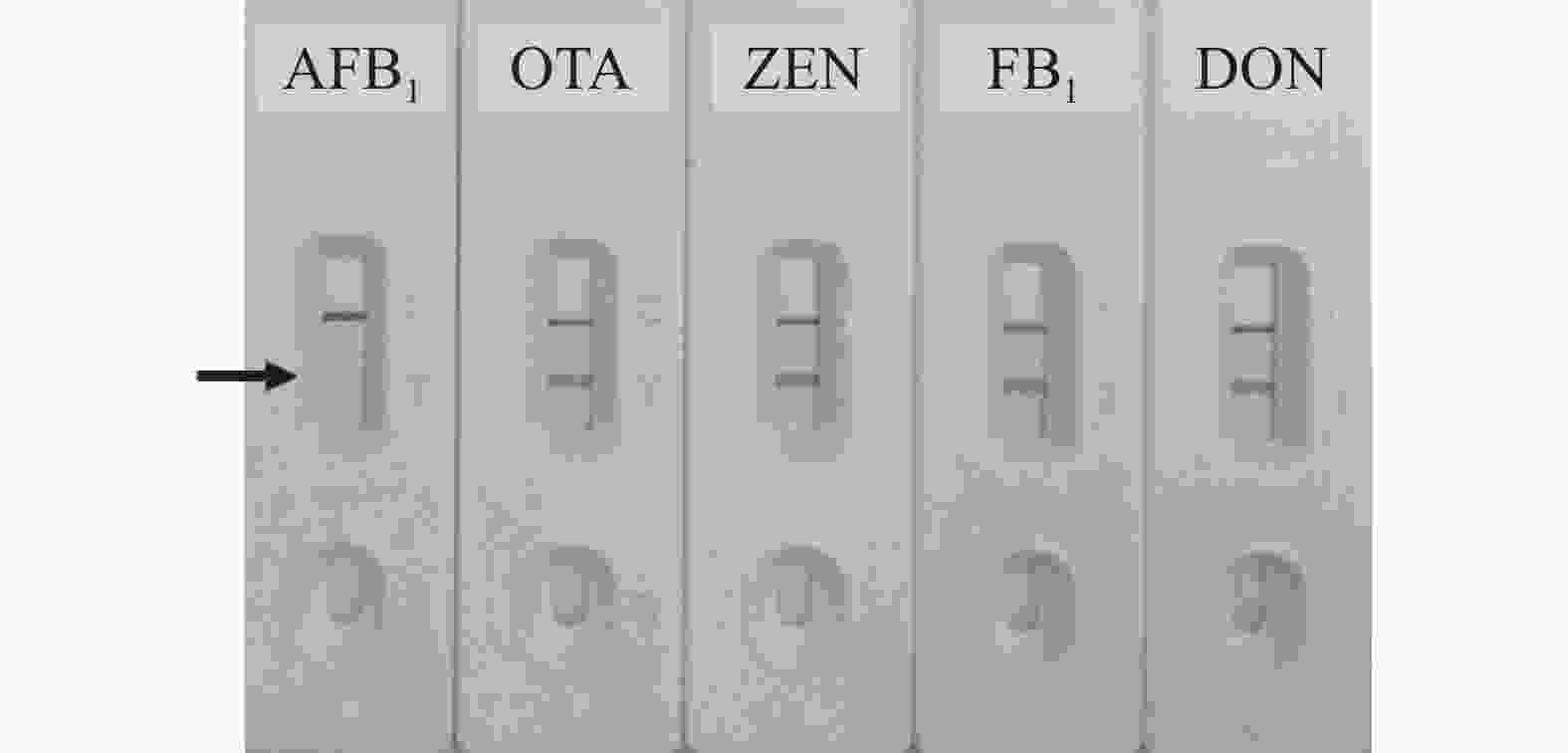
以谷物类样本中其他常见真菌毒素如赭曲霉毒素A(OTA)、玉米赤霉烯酮(ZEN)、伏马毒素B1 (FB1)和呕吐毒素(DON)作为竞争抗原,进行特异性验证,结果如图7所示,建立的免疫层析检测法对上述毒素均不存在交叉反应,特异性较好,质量浓度均为5.00 μg·L−1。
-
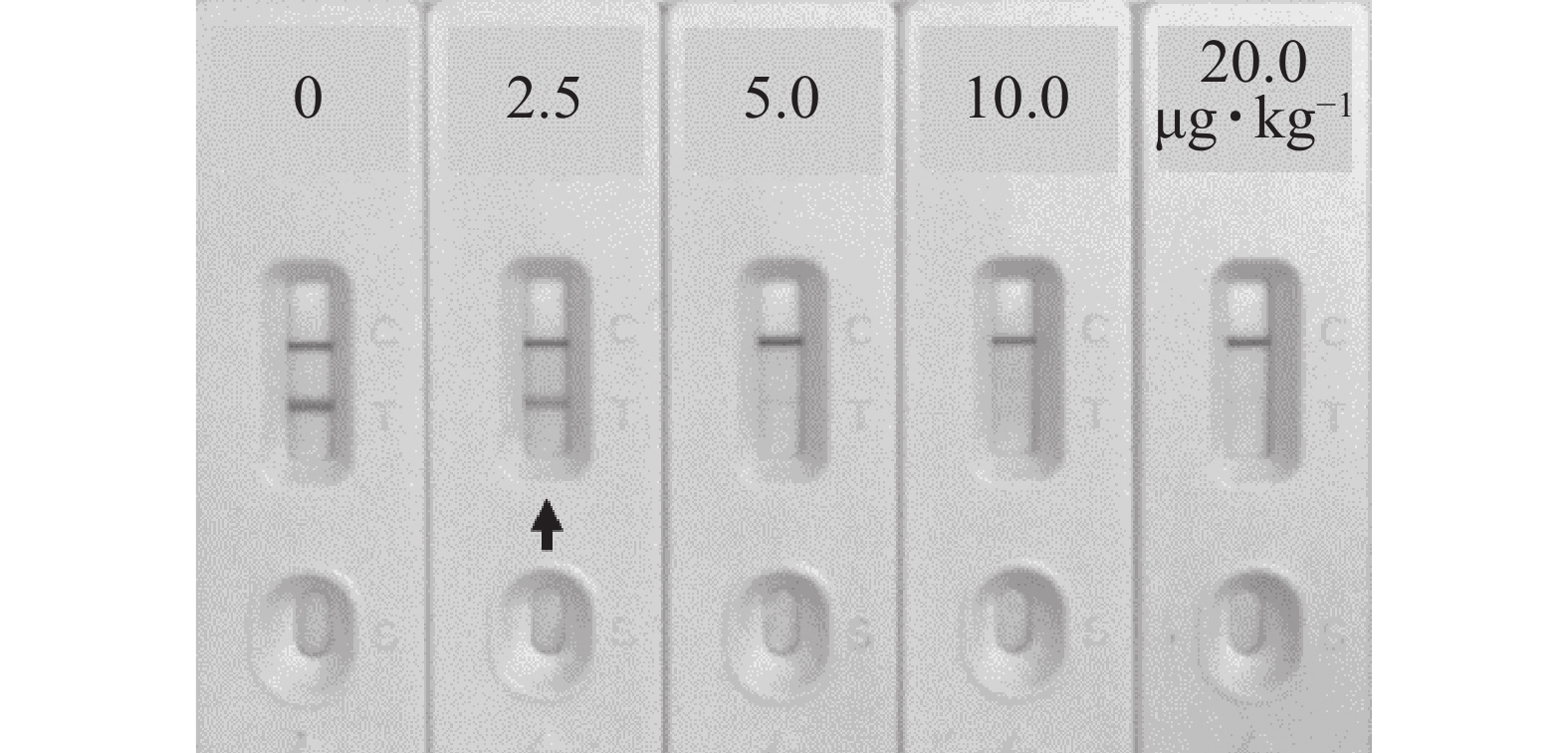
如图8所示:对玉米Zea mays样本进行加标试验,当AFB1加标质量分数为2.5 μg·kg−1可使检测线变化明显,说明该免疫层析检测法在实际样本中的定性检测限为2.5 μg·kg−1。
当AFB1加标质量分数依次为1.0、2.5、5.0和15.0 μg·kg−1时,如表1所示,该免疫层析法在玉米样本中的加标回收率为89.62%~110.43%,批内变异系数为4.51%~6.58%,批间变异系数为7.28%~9.72%,说明该方法准确率较高且稳定性较好。
AFB1加标质量
分数/(μg·kg−1)批内 批间 回收
率/%变异系
数/%回收
率/%变异系
数/%1.0 89.62 ± 5.31 5.93 95.72 ± 8.03 8.39 2.5 96.47 ± 6.35 6.58 103.56 ± 7.54 7.28 5.0 95.16 ± 4.29 4.51 93.25 ± 9.06 9.72 10.0 110.43 ± 6.15 5.57 107.59 ± 8.56 7.96 说明:数据为平均值±标准差,n=3 Table 1. Quantitative detection of aflatoxin B1 spiked samples by immunochromatography assay
-
37 ℃加速稳定性实验结果表明:建立的免疫层析试纸条放置30 d后仍能对AFB1进行定性检测与定量分析,灵敏度未受影响,表明稳定性良好,推测室温可稳定保存1 a。
-
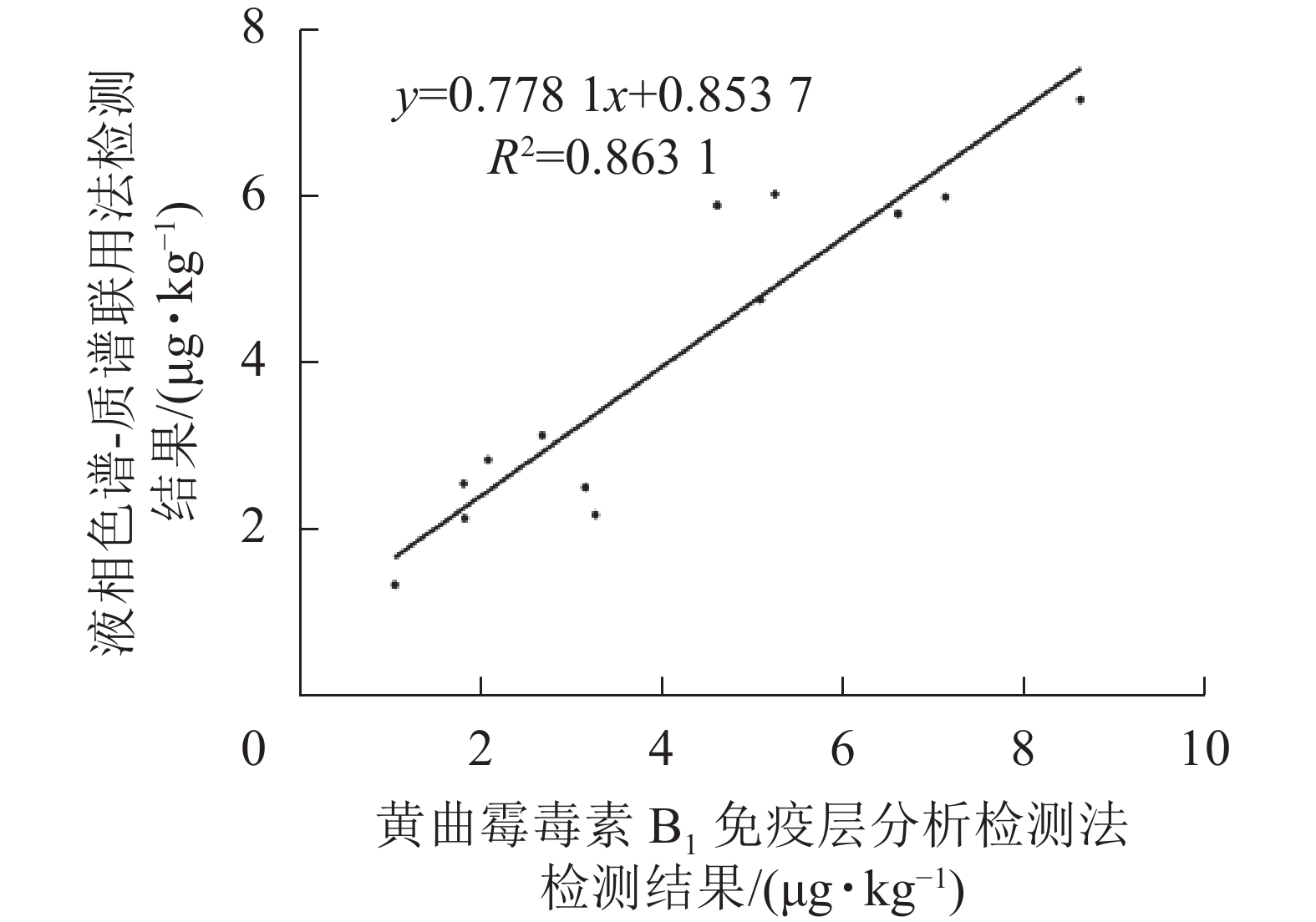
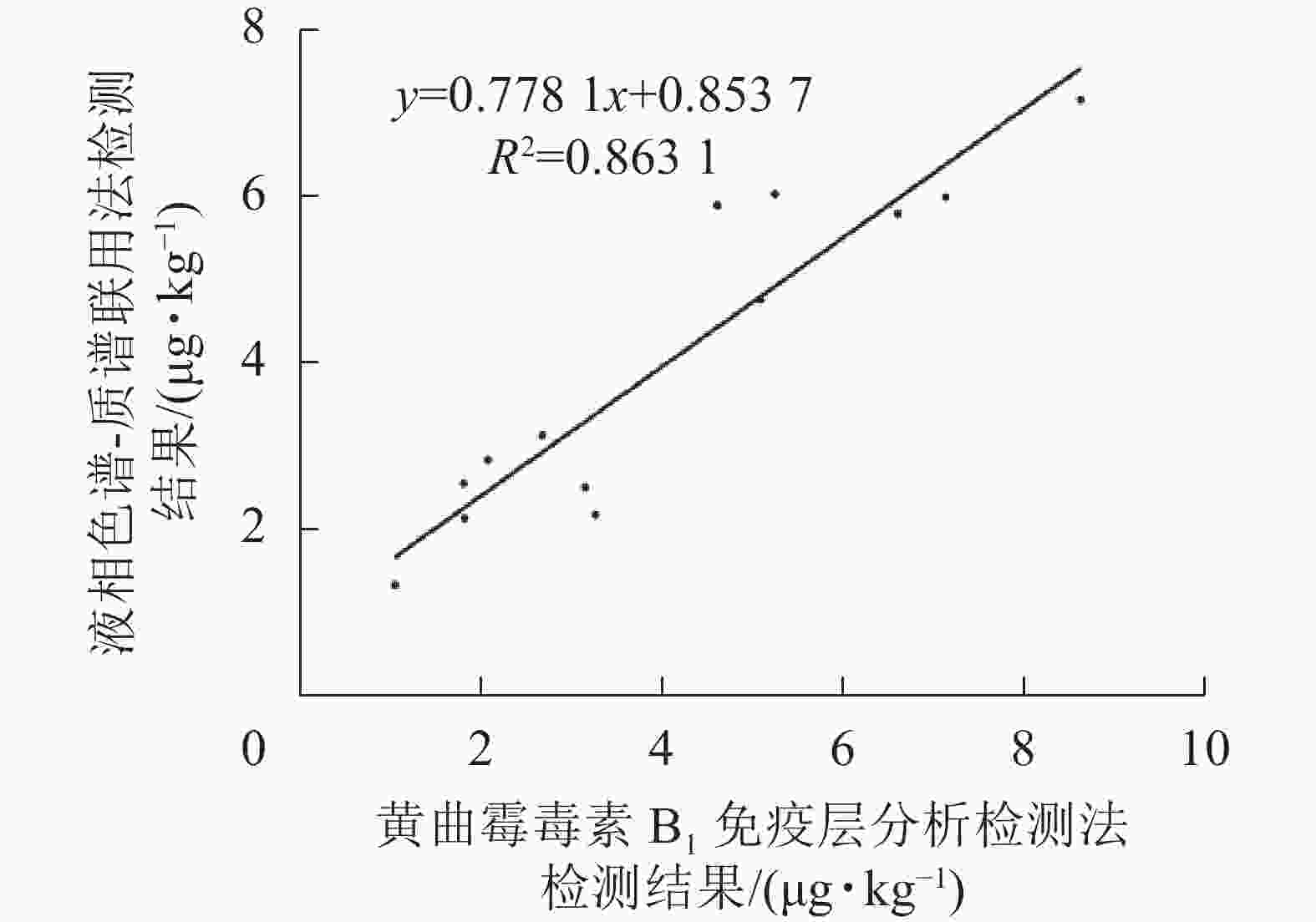
如表2和图9所示:免疫层析法检测结果与LC-MS/MS的相关性一致性较好(R2=0.863 1),与商品化试剂盒的检测结果经SPSS软件分析,同样显示显著相关(P<0.01)。综上表明:本研究建立的免疫层析检测法可适用于实际样本中AFB1的快速定量检测与分析。
样本编号 AFB1/(μg·kg−1) 样本编号 AFB1/(μg·kg−1) 免疫层析检测法 LC/MS/MS 商品化试剂 免疫层析检测法 LC/MS/MS 商品化试剂 1 5.11 ± 0.32 4.79 ± 0.29 5.65 ± 0.37 8 1.83 ± 0.14 2.15 ± 0.27 2.71 ± 0.19 2 3.17 ± 0.25 2.52 ± 0.16 2.08 ± 0.18 9 2.09 ± 0.17 2.86 ± 0.25 3.57 ± 0.26 3 1.06 ± 0.11 1.34 ± 0.14 2.33 ± 0.32 10 8.65 ± 0.35 7.21 ± 0.62 8.94 ± 0.19 4 6.63 ± 0.47 5.83 ± 0.43 5.36 ± 0.57 11 4.63 ± 0.29 5.93 ± 0.38 4.38 ± 0.23 5 2.69 ± 0.22 3.15 ± 0.29 2.25 ± 0.26 12 5.27 ± 0.41 6.07 ± 0.57 5.66 ± 0.35 6 1.82 ± 0.13 2.57 ± 0.15 3.35 ± 0.18 13 3.28 ± 0.37 2.19 ± 0.18 3.95 ± 0.31 7 7.16 ± 0.51 6.03 ± 0.31 7.82 ± 0.23 说明:数据为平均值±标准差 Table 2. Quantitative detection of AFB1 in natural samples by the developed immunochromatography assay, commercial ELISA kit and LC-MS/MS
-
常规免疫层析检测法采用胶体金颗粒作为抗体标记物,通过在检测线和质控线形成明显的颜色反应,因此金颗粒粒径对抗体标记效率和检测灵敏度影响较大。本研究中,40 nm金颗粒标记AFB1单克隆抗体后,与20 nm相比,虽在检测灵敏度上无明显优势,但稳定性更佳,故确定为最终的抗体标记物。作为免疫层析系统的重要组成部分,不同类型硝酸纤维素膜、金标抗体固定垫和样品垫,会直接影响检测效果[21]。本研究经对比,选用Sartorius CN 140、聚酯膜Ahlstrom 8964和玻璃纤维SB08分别作为硝酸纤维素膜、金标抗体固定垫和样品垫使用。层析体系中各缓冲液的种类和质量浓度同样影响检测效果,如检测线或质控线信号强度和清晰度、金标抗体释放效率和稳定性以及样本在硝酸纤维素膜上的迁移速率等[23-24],并且适量质量分数蔗糖/海藻糖、BSA、PEG 2000、NaN3以及表面活性剂TritonX-100或Tween-20的加入有助于获得更好的检测效果和稳定性[23-26]。本研究制备的AFB1免疫层析检测法在实际样本中的定性和定量检测限分别为2.5和0.5 μg·kg−1,满足谷物及饲料中AFB1快速定性检测和定量分析需求。相比ELISA,该定性定量免疫层析法更加简单快速且成本低,适用于大量样本的快速初筛,样本结果疑似阳性再选用仪器法进行确认分析,可大大提升检测效率。
A highly sensitive qualitative and quantitative immunochromatographic method for the detection of aflatoxin B1
doi: 10.11833/j.issn.2095-0756.20210772
- Received Date: 2021-11-29
- Accepted Date: 2022-04-01
- Rev Recd Date: 2022-03-31
- Available Online: 2022-09-22
- Publish Date: 2022-10-20
-
Key words:
- qualitative detection /
- quantitative analysis /
- aflatoxin B1 /
- immunochromatography /
- monoclonal antibody
Abstract:
| Citation: | ZHANG Xian, WANG Jixuan, CHENG Gaochuan, et al. A highly sensitive qualitative and quantitative immunochromatographic method for the detection of aflatoxin B1[J]. Journal of Zhejiang A&F University, 2022, 39(5): 1096-1103. DOI: 10.11833/j.issn.2095-0756.20210772 |














 DownLoad:
DownLoad: