-
油茶Camellia oleifera是山茶科Theaceae山茶属Camellia常绿小乔木或大灌木[1],它与油桐Vernicia fordii、核桃Juglans regia、乌桕Triadica sebifera并称为“中国四大木本油料树种”。油茶栽培历史悠久,种植面积广,主要分布在中国南方丘陵地区,如湖南、江西、安徽和贵州等。油茶种子可提取出茶油,茶油营养价值高,且含有不饱和脂肪酸,符合现代人对食用油的健康需求[2]。目前,中国种植的油茶约73%是传统种子杂交育种,性状不稳定,育种周期长,且基因组杂合程度高,很有可能发生子代遗传变异[3]。
植物组织培养中单倍体育种方法可以快速筛选有优良性状的纯合子植株,加快优良品种繁育进程。单倍体育种可以分为2种:离体雌核和雄核诱导。雌核指的是植物的大孢子体(子房或胚珠),雄核指的是小孢子体(花药或花粉)。在油茶的单倍体育种研究方面,前人针对油茶花药开展离体培养,探索愈伤组织诱导的影响因素等[4-6],但目前尚未获得再生植株,另外,靳皓然等[7]对油茶未授粉子房进行了愈伤组织诱导。近年来,未授粉雌核培养已作为一种获得单倍体的有效手段被广泛应用[8],其中植物未授粉胚珠离体培养获得单倍体再生植株已在多种草本植物和少数木本植物中取得成功,如万寿菊Tagetes erecta [9]、甜菜Beta vulgaris [10]、柑橘Citrus reticulata [11]等,关于油茶未授粉胚珠离体培养尚未见报道。
愈伤组织在诱导过程中会出现非胚性和胚性愈伤组织2种形态,两者外观差异较大。非胚性愈伤组织大多呈不规则球形,质地疏松、表面粗糙。胚性愈伤组织形态因物种不同而差异较大,或为浅绿色、表面有密集球状突起、质地较为紧密,或为黄白色颗粒状、团簇状,或呈黄褐色且质地疏松等[2, 12-13]。
本研究对油茶离体雌核培养中的未授粉胚珠进行了愈伤组织诱导,这是单倍体培养的第一阶段,即胚性愈伤组织的诱导,得到了较为稳定高效的油茶未授粉胚珠胚性愈伤组织诱导技术体系,为进一步建立完整高效的油茶体细胞胚胎发生和单倍体育种体系提供参考。
-
以油茶‘华金’C. oleifera‘Huajin’的未授粉胚珠为外植体。花苞取自湖南省长沙市望城区大果油茶技术集成示范园(112°50′38″N,28°30′39″E)和岳麓区油茶新品种产学研创新基地(112°52′12″N,28°03′09″E)。于2021年11—12月天气晴朗的9:00—11:00采集未授粉花蕾,放入冰盒中带回实验室,当天进行接种。
-
将采集的油茶未授粉花蕾置于流水下冲洗20 min,用镊子剥除花瓣、花萼、花药、花柱等部分,仅剩余子房。全部放入无菌操作台中用无菌水冲洗3~5遍,先用体积分数为75%的乙醇浸泡消毒1 min,无菌水冲洗3~5遍,再用质量分数为0.1%的氯化汞溶液浸泡消毒5 min,期间不断振荡,最后用无菌水冲洗3~5遍[7]。将消毒后的子房用滤纸吸干水分,纵向居中切割成两半,用一次性1 mL医用注射器的针尖小心地将胚珠从子房中挑出,接种到培养基上。
诱导愈伤组织培养基由基础培养基MS培养基(Murashig and Skoog medium, MS)、木本植物用培养基(Lloyd&Mc Cown woody plant basal medium with vitamin, WPM)、MT培养基(Murashige tucker medium, MT)、1/2 MS培养基、B5培养基、蔗糖,以及生长素萘乙酸(NAA)、吲哚乙酸(IAA)、吲哚丁酸(IBA)、二氯苯氧乙酸(2,4-D)和细胞分裂素苄氨基嘌呤(6-BA)、激动素(KT)、噻苯隆(TDZ)组成(以上药品均购自于上海市索莱宝科技有限公司),并用7 g·L−1琼脂固化,1 mol·L−1盐酸溶液或氢氧化钠溶液将培养基pH调整到5.4~5.6,置于121 ℃高温高压灭菌锅中灭菌20 min,在无菌操作台中迅速分装到直径为9 cm的一次性圆形培养皿中,每皿25 mL。
-
采用逐步筛选的单因素试验设计,首先选择5种基础培养基(MS、WPM、MT、1/2MS、B5),加入1.0 mg·L−1 2,4-D和2.0 mg·L−1 6-BA,50 g·L−1蔗糖和7 g·L−1琼脂,筛选出诱导愈伤组织合适的基础培养基;再选择上述试验筛选出的基础培养基,分别将1.0 mg·L−1生长素(NAA、IAA、IBA、2,4-D)和2.0 mg·L−1细胞分裂素(6-BA、KT、TDZ)两两组合,添加50 g·L−1蔗糖和7 g·L−1琼脂,筛选出合适的植物生长调节剂组合;以上述合适的2种植物生长调节剂和蔗糖作为因素,设计三因素三水平正交试验,其中生长素(0.5、1.0、2.0 mg·L−1),细胞分裂素(1.0、2.0、3.0 mg·L−1),蔗糖(40、50、60 g·L−1),筛选出诱导愈伤组织合适的培养基配方。
每个培养皿中接种36颗胚珠,每组处理接种3皿,重复3次。接种后的胚珠首先置于黑暗条件下培养7 d,再转入光照培养室中培养,条件设置为(25±1) ℃,光照/黑暗周期为16 h/8 h,光照强度为36 μmol·m−2·s−1。培养30 d后,统计未授粉胚珠的愈伤组织诱导率和褐化率。
-
在无菌操作台中取出愈伤组织,在体视显微镜(Olympus-DP73)下进行形态学观察并拍照。
石蜡切片制作方法参照陈乐[14]并部分调整,将诱导出的愈伤组织放入卡诺氏固定液[V(无水乙醇)∶V(冰醋酸)=3∶1]中固定10 h。固定好的愈伤组织在乙醇中逐级脱水,后放入1/2二甲苯[V(无水乙醇)∶V(二甲苯)=1∶1]、纯二甲苯中进行透明,加入1/2石蜡后放入37 ℃烘箱中浸蜡48 h,再转入60 ℃烘箱中浸蜡72 h,使石蜡充分浸入组织中。用纯石蜡将材料包埋,冷却凝固后用德国徕卡(Leaka RM2235)切片机切成厚度10 μm的石蜡带,在蒸馏水中展片后放入卧式玻璃染缸中脱蜡。脱蜡后加入质量分数为0.5%的硫酸铁铵溶液媒染15 min后用流水冲洗1 h。选择苏木精和曙红为染色液染色,再逐级复水,最后用中性树胶封片。将制作好的永久切片在电子显微镜(Olympus-BX53)进行细胞学观察并拍照。
-
以油茶‘华金’无菌苗的幼嫩叶片为参照,将诱导出的愈伤组织进行倍性鉴定。选取大约0.5 cm3的愈伤组织块放入培养皿中,加入UV Precise P 细胞核提取液200 μL,用双面刀片切成碎末状,再加入200 μL提取液制成细胞悬液。用30 μm孔径的滤嘴过滤到滤管中,加入1 600 μL UV Precise P 染色液避光染色5 min后,用流式细胞仪(Sysmex)检测5 000~10 000个细胞的倍性[6, 15]。
-
诱导率=诱导出愈伤组织的胚珠数/接种的胚珠数×100%;褐化率=褐化的胚珠或愈伤组织数/接种的胚珠数×100%;使用Adobe Photoshop CC 2018处理照片,Origin 2021作图,Excel 2010和SPSS 26.0进行数据统计和显著性分析。
-
MS培养基作为基础培养基时胚珠的愈伤组织诱导率最高,为72.50%,1/2 MS、WPM和B5培养基次之,诱导效果最差的是MT培养基,诱导率仅26.30%,且MT培养基上褐化率最高,达82.17%,表明MT培养基不适合胚珠诱导愈伤组织(表1)。在MS培养基上愈伤组织的褐化率最低,为21.65%,1/2 MS和B5培养基稍差,褐化率分别为26.35%、28.87%。其中MS和1/2 MS培养基的愈伤组织诱导率和褐化率,都优于其他基础培养基。因此MS是最适合油茶未授粉胚珠诱导愈伤组织的基础培养基。
编号 培养基类型 诱导率/% 褐化率/% 1 MS 72.50±5.15 a 21.65±1.88 d 2 WPM 69.16±1.74 a 37.38±1.02 b 3 MT 26.30±3.44 c 82.17±0.27 a 4 1/2 MS 71.18±4.49 a 26.35±5.62 cd 5 B5 50.19±6.64 b 28.87±0.64 c 说明:不同字母表示差异显著(P<0.05)。 Table 1. Callus induction of C. oleifera ovule under different basal mediums
-
以2.1中筛选出的MS为基础培养基,选择4种生长素(1.0 mg·L−1)和3种细胞分裂素(2.0 mg·L−1)两两组合,研究适合胚珠诱导愈伤组织的植物生长调节剂组合(表2)。由表2可知:诱导效果最佳的植物生长调节剂组合是NAA+TDZ,诱导率为88.89%,IBA+TDZ组合次之,为85.75%,另一组使用TDZ的组合是IAA+TDZ,诱导率为69.72%,列第4位。
编号 生长素质量浓度/(mg·L−1) 细胞分裂素及质量浓度/(mg·L−1) 诱导率/% 褐化率/% NAA IAA IBA 2,4-D 6-BA KT TDZ 1 1.0 0 0 0 2.0 0 0 26.92±1.92 de 54.33±25.99 bc 2 1.0 0 0 0 0 2.0 0 52.05±11.53 c 34.02±4.24 cd 3 1.0 0 0 0 0 0 2.0 88.89±5.16 a 11.11±2.34 e 4 0 1.0 0 0 2.0 0 0 30.83±7.89 d 91.76±2.14 a 5 0 1.0 0 0 0 2.0 0 30.90±5.90 d 76.83±9.85 a 6 0 1.0 0 0 0 0 2.0 69.72±9.08 b 48.68±14.33 bc 7 0 0 1.0 0 2.0 0 0 18.85±1.55 e 88.45±2.25 a 8 0 0 1.0 0 0 2.0 0 37.70±3.88 d 69.83±10.15 ab 9 0 0 1.0 0 0 0 2.0 85.75±5.17 a 47.70±5.95 c 10 0 0 0 1.0 2.0 0 0 72.50±1.55 c 21.65±5.18 de 11 0 0 0 1.0 0 2.0 0 60.04±1.15 bc 42.59±14.37 cd 12 0 0 0 1.0 0 0 2.0 55.94±4.05 c 39.69±5.16 cd 说明:不同字母表示差异显著(P<0.05)。 Table 2. Callus induction of C. oleifera ovary under different hormone combinations
4组使用TDZ作为细胞分裂素的组合处理下的褐化率差异较大,可能是生长素的种类不同导致愈伤组织的褐化程度不同。由表2可得:添加NAA的3组诱导率均值为33.15%,添加IAA、IBA、2,4-D的诱导率均值分别为72.42%、68.66%和34.64%。可见,添加NAA作为生长素的愈伤组织褐化率低于其他组合,其中NAA+TDZ组合的褐化率最低,为11.11%,较适合愈伤组织的诱导。添加IAA或IBA的6组植物生长调节剂组合褐化率均较高,添加2,4-D的10、11和12组的褐化率均值为34.64%,略高于使用NAA的1、2和3组的褐化率均值33.15%。因此,植物生长调节剂组合对胚珠愈伤组织诱导和褐化均有显著影响(P<0.05),生长素和细胞分裂素都对诱导率有影响,其中细胞分裂素影响较大;生长素对褐化率影响较为显著,NAA+TDZ组合的愈伤组织诱导率最高为88.89%,褐化率最低仅11.11%,为最佳激素组合。
-
根据2.2中筛选出的植物生长调节剂组合NAA+TDZ,设计三因素三水平正交试验并进行极差分析,研究NAA、TDZ和蔗糖诱导胚珠愈伤组织的最适质量浓度,以及它们的影响顺序(表3)。其中NAA质量浓度为0.5、1.0、2.0 mg·L−1,TDZ质量浓度为1.0、2.0、3.0 mg·L−1,蔗糖质量浓度为40、50、60 g·L−1。由表3可知:诱导效果最佳的处理为2.0 mg·L−1 NAA+2.0 mg·L−1 TDZ+40 g·L−1蔗糖,诱导率为92.97%,其次为1.0 mg·L−1 NAA+2.0 mg·L−1 TDZ+60 g·L−1蔗糖和1.0 mg·L−1 NAA+1.0 mg·L−1 TDZ+50 g·L−1蔗糖,诱导率分别为89.11%和85.65%。极差分析显示:TDZ对诱导率的影响程度最大,诱导率极差(R)为15.46,NAA次之(诱导率极差为12.37),蔗糖的影响程度最小,诱导率极差为4.99。
编号 植物生长调节剂质量浓度/(mg·L−1) 蔗糖质量浓度/(g·L−1) 愈伤组织诱导率/% 褐化率/% 愈伤组织形态 NAA TDZ 1 0.5 1.0 40 70.65±7.62 bc 50.09±8.61 ab 质地疏松,黄褐色 2 0.5 2.0 50 71.26±12.06 bc 36.01±13.26 ab 质地疏松,浅黄色 3 0.5 3.0 60 68.90±16.16 bc 54.10±1.47 a 质地疏松,乳白色或黄褐色 4 1.0 1.0 50 85.65±7.65 abc 33.79±11.81 b 质地紧密,黄白色 5 1.0 2.0 60 89.11±2.46 ab 10.81±5.16 c 质地紧密,浅绿色 6 1.0 3.0 40 73.15±15.61 abc 48.26±10.57 ab 质地紧密,乳白色 7 2.0 1.0 60 71.44±11.95 bc 46.30±5.26 ab 质地疏松,黄褐色 8 2.0 2.0 40 92.97±5.97 a 34.26±12.90 b 质地紧密,黄色 9 2.0 3.0 50 64.92±2.42 c 52.43±12.15 ab 质地疏松,浅黄色或黄褐色 $\overline {X}_1$ 70.27 75.91 78.93 $\overline {X}_2$ 82.64 84.45 73.94 $\overline {X}_3$ 76.44 68.99 76.48 R 12.37 15.46 4.99 说明:不同字母表示差异显著(P<0.05)。表中$\overline X_1 $、$\overline X_2 $、$\overline X_3 $代表正交试验的3个水平,R为诱导率极差。 Table 3. Induction rate of C. oleifera ovary callus in the L9 (33) orthogonal test of hormone and sucrose combination
3个因素的最佳水平分别为NAA($\overline X_2$=82.64%)、TDZ($\overline X_2 $=84.45%)、蔗糖($\overline X_2 $=78.93%),因素的影响从大到小依次为TDZ、NAA、蔗糖。诱导胚珠愈伤组织的最适组合为1.0 mg·L−1 NAA+2.0 mg·L−1 TDZ+40 g·L−1蔗糖。
-
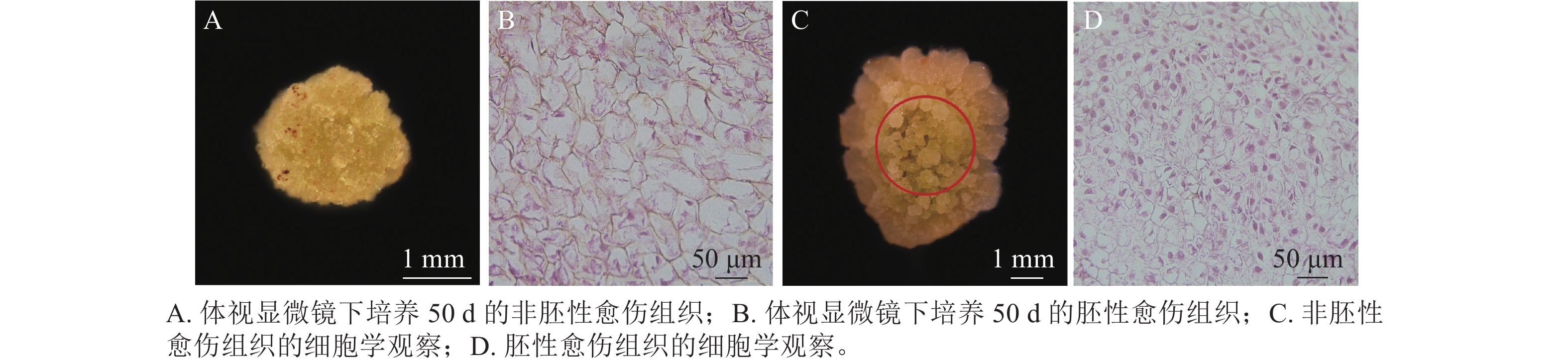
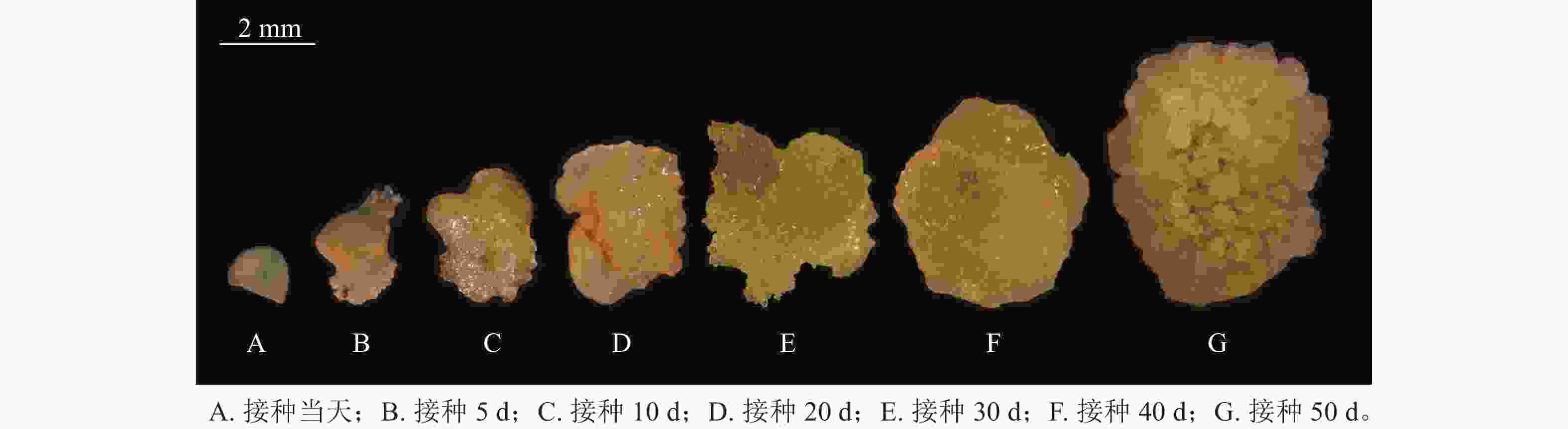
在胚珠愈伤组织的诱导过程中发现了2种愈伤组织形态,即非胚性愈伤组织(non-embryonic callus)和胚性愈伤组织(embryonic callus)。其中非胚性愈伤组织取自表1中的B5培养基,培养基配方为B5 +1.0 mg·L−1 2,4-D和2.0 mg·L−1 6-BA+50 g·L−1蔗糖+7 g·L−1琼脂,培养至50 d的形态如图1A所示。非胚性愈伤组织为不规则球形,质地疏松,表面粗糙并有少量凸起,外观呈嫩绿稍偏黄色。石蜡切片显微观察显示其细胞核小,液泡占据整个细胞面积85%以上,细胞形态不规则,排列松散,容易破碎,基本无分裂能力(图1B)。
培养至50 d的胚性愈伤组织取自表3中的5组(图1C),培养基配方为MS+1.0 mg·L−1 NAA和2.0 mg·L−1TDZ+60 g·L−1蔗糖+7 g·L−1琼脂。胚性愈伤组织质地紧密,呈浅绿色,体视显微镜下可看到明显由内向外的密集球状凸起。显微结构(图1D)显示其细胞排列紧密,大小一致,细胞核染色面积大,细胞质浓厚,分生能力较强,胚性细胞团为后续愈伤组织分化成胚状体提供可能。
图2是在体视显微镜下跟踪观察并拍摄的接种当天至培养50 d的愈伤组织生长过程中的外部形态变化。可以看出前期形成的是非胚性愈伤组织,在50 d左右逐渐转变为胚性愈伤组织。
-
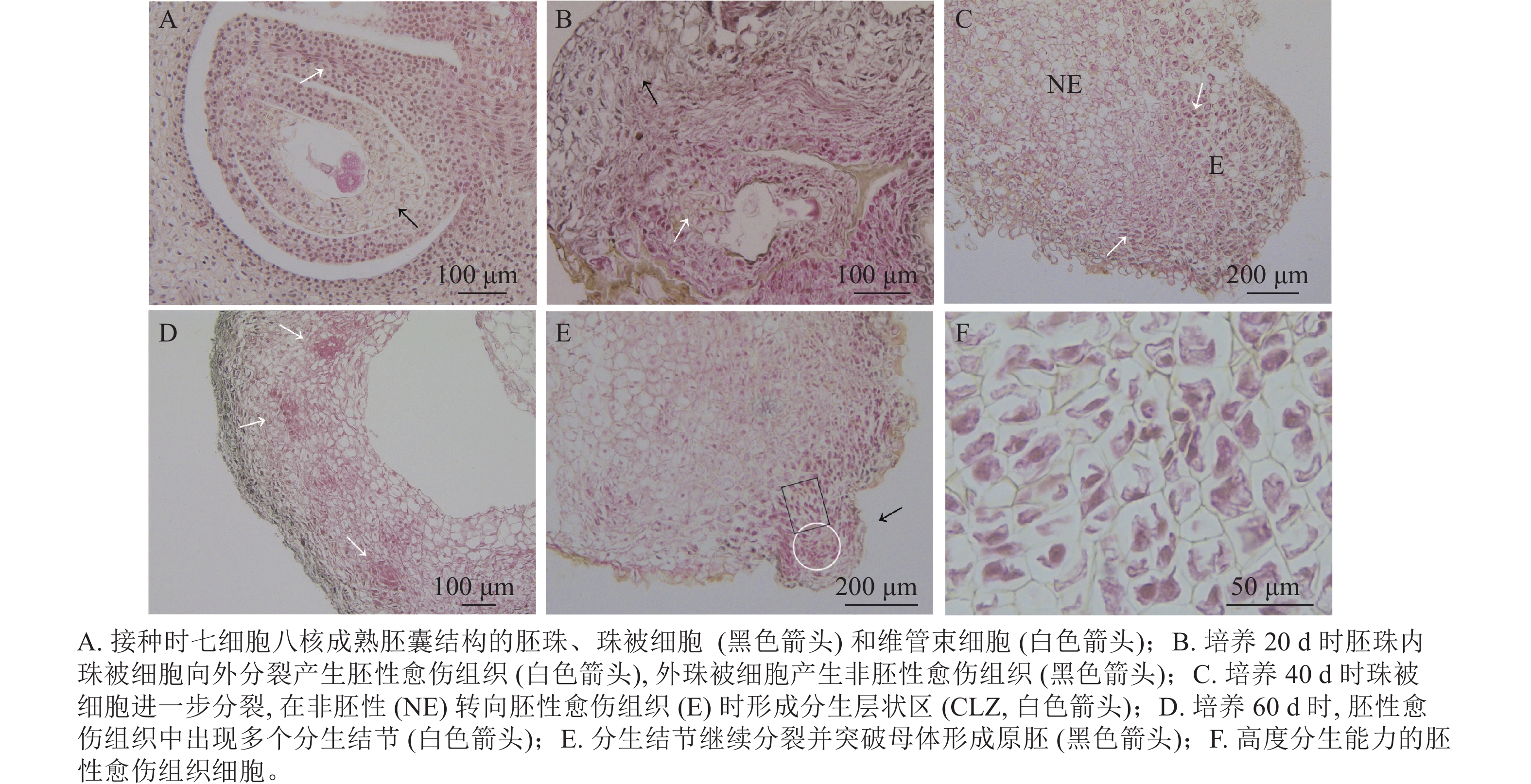
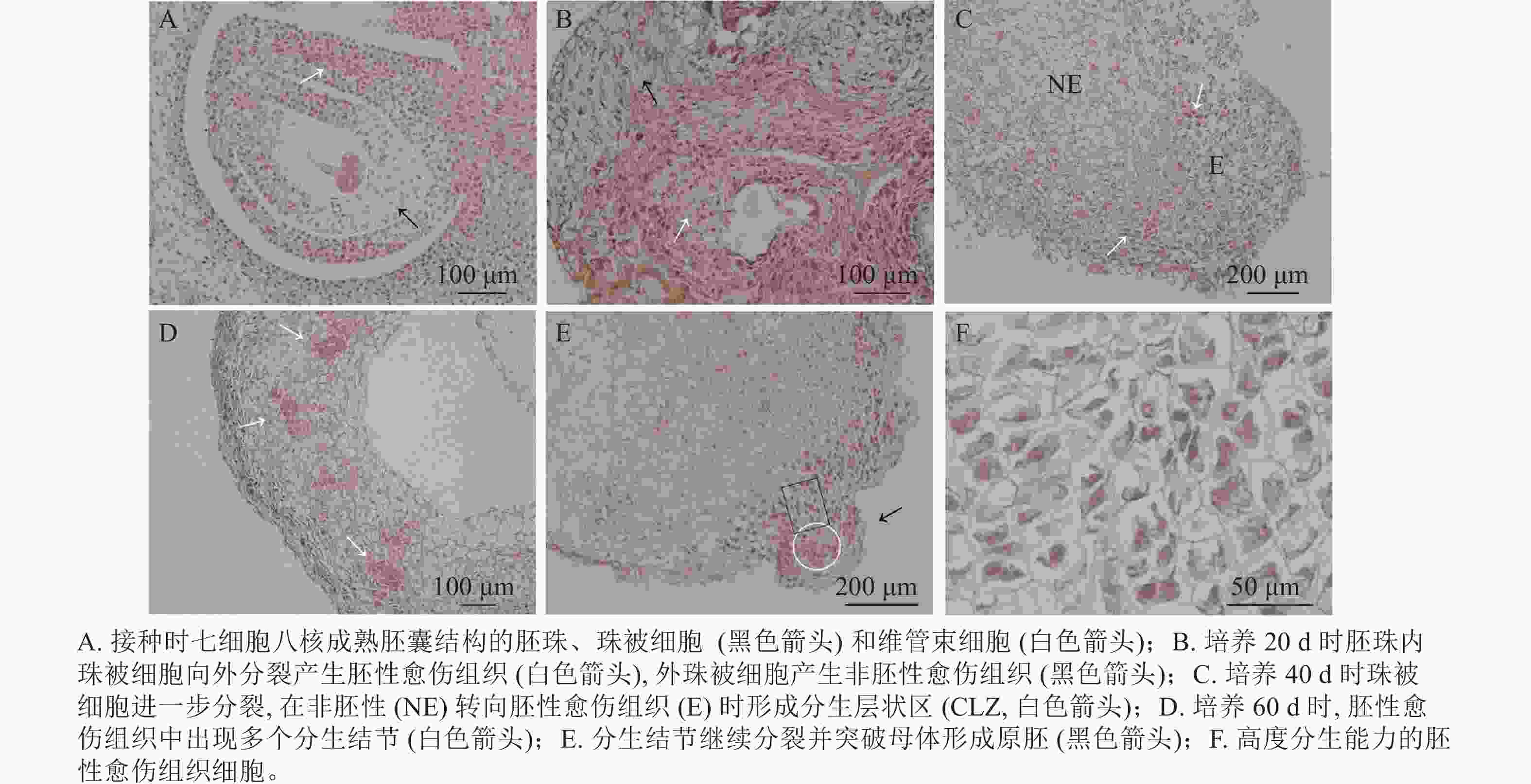
油茶未授粉胚珠接种当天,在电子显微镜下可看到七细胞八核成熟胚囊时期的胚珠结构[16],细胞核被染为蓝黑色,细胞质为淡粉色(图3A)。珠被表皮细胞处于高度分化状态,细胞形态规则,大小一致,液泡较大(黑色箭头所示);维管束细胞体积较小,排列紧密(白色箭头所示)。愈伤组织起源于多个细胞同时进行的分裂增殖(图3B),培养20 d时在外界诱导作用的影响下,胚珠内珠被细胞从合点端向外分裂并向外突出,细胞排列紧密,且细胞核较大,为胚性愈伤组织(图3B白色箭头所示)。同时外珠被细胞、维管束细胞等也开始进行分裂增殖,形成结构疏松的非胚性愈伤组织(图3B黑色箭头所示)。但内珠被细胞由于增殖空间有限而逐渐停止分裂,外珠被细胞继续分裂生长。培养40 d时,珠被细胞部分区域出现非胚性细胞(图3C中NE部分)转为胚性细胞(图3C中E部分)的现象,细胞整体缩小而细胞核变大,进一步向外分裂增殖,形成1个相对独立的高度分生的胚性细胞条带,这一部分在PERERA等[17]的研究中被称为形成层状区(cambium-like zone, CLZ)(图3C,白色箭头所示)。培养60 d时,珠被细胞的胚性细胞团进一步分裂增殖,形成层状区被破坏,分生结节(meristematic nodules, Mn)突出原细胞团而产生(图3D白色箭头所示),即数个胚性细胞组成1个较为独立的细胞团,这部分可进一步分化为胚状体(图3D)。分生结节进一步向表皮方向生长,并突出原表皮细胞而形成原胚(图3E中黑色箭头所示),组成原胚的细胞有2种:一是头部的体积较小,近方形的“胚头”细胞(图3E白色圆形框内),此种细胞的分裂能力很强,突出原愈伤组织而独立即是这部分细胞完成的;二是长条形的“胚柄”细胞(图3E黑色矩形框内),当“胚头”细胞脱离母体后“胚柄”细胞仍与原愈伤组织连接。原胚由高度分生能力的胚性细胞组成,细胞核较大,细胞壁薄,排列紧密无间隙,既可以继续分裂也具有分化能力,在图3F中可以看到不同分裂时期的细胞。
由此可见,油茶未授粉胚珠诱导的愈伤组织来源于珠被细胞的多细胞分裂,内珠被细胞分裂受阻停止生长,外珠被细胞首先脱分化形成疏松的非胚性细胞团,之后部分细胞的细胞核变大,排列紧密,转变为胚性细胞团,胚性细胞团进一步分裂增殖并形成独立分生结节,后脱离原细胞团形成原胚,是为胚状体形成的原始结构。
-
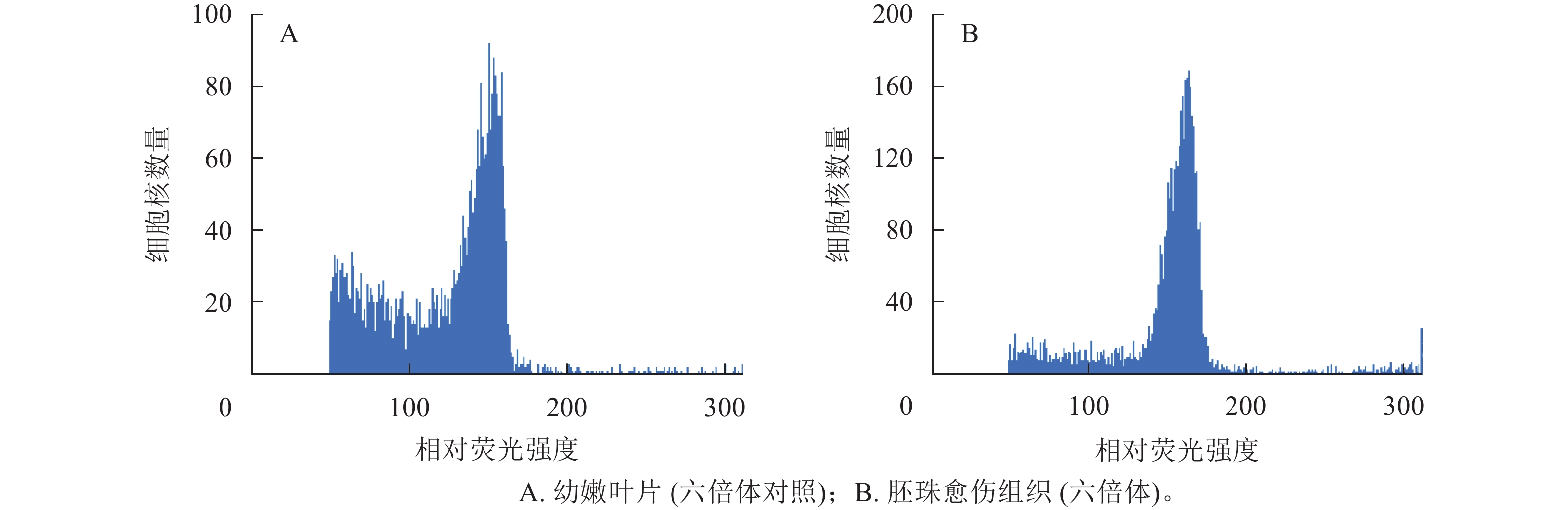
以油茶‘华金’的幼嫩叶片为对照(图4A),对诱导出的愈伤组织进行倍性鉴定,随机选取愈伤组织共96份,鉴定结果显示:96份愈伤组织倍性均为六倍体(图4B),与叶片倍性一致,说明愈伤组织的来源一般为珠被的体细胞,这与2.5中的愈伤组织细胞来源于外珠被细胞的分析结果一致。
-
基础培养基的筛选是植物组织培养的关键一环,目前在愈伤组织诱导培养中常见的基础培养基约有14种[18]。本研究选取MS、WPM、MT、1/2 MS、B5等5种基础培养基进行研究,其中MS培养基是应用较普遍的一种,如彭秋发等[19]对攸县油茶成熟子叶诱导胚性愈伤组织的研究表明:MS作为基本培养基,添加6-BA和NAA可以诱导产生胚状体;范晓明等[5]在油茶花药诱导愈伤组织时选择了MS作为基本培养基;油茶未成熟幼胚诱导胚状体中选择MS作为胚启动培养基[2]等。MS培养基的硝酸盐浓度较高,养分比例合适,可以满足茎尖、子叶、幼胚等多种外植体的营养物质需求,本研究中,1/2 MS培养基的愈伤组织诱导率也较高,推测两者培养基中的无机盐、营养物质等比例较为合适,能够满足胚珠诱导愈伤组织的需要。但袁素霞等[20]认为:高浓度氮的培养基可能会对未授粉雌核的培养有不利影响,因此1/2 MS、WPM和B5等含有较低浓度NH4 +的培养基在一些植物中有较好的应用效果[21−24]。韩春霞等[6]在油茶花药愈伤组织诱导中也认为处理为低浓度NH4 +的MS培养基效果较好。但在本研究中MS培养基的诱导率与WPM培养基诱导率差异不显著;而同为低浓度NH4 +的WPM、B5培养基却差异显著,诱导率相差18.97%,因此推测NH4 +浓度并不是影响油茶未授粉胚珠诱导率的关键因素。MT培养基在柑橘未授粉胚珠诱导再生植株上取得成功[11],但MT培养基在本研究中诱导率仅为26.30%,并不适用于油茶未授粉胚珠诱导愈伤组织。MS、1/2 MS和WPM培养基在愈伤组织诱导率方面均表现良好,其中MS培养基的愈伤组织诱导率最高,且褐化率为三者最低,仅21.65%,因此MS培养基是油茶未授粉胚珠诱导愈伤组织最适合的基础培养基。
植物生长调节剂在愈伤组织诱导中起到调控细胞分裂分化的关键作用[25],高度分化的细胞处于分裂静止状态,植物生长调节剂可以打破细胞的抑制物质,使之重新开始分裂活动,进入脱分化时期。本研究发现:生长素和细胞分裂素对愈伤组织诱导都有显著影响,后者的影响程度更大。细胞分裂素TDZ是胚珠愈伤组织诱导中的关键植物生长调节剂,同样的结果也在黄瓜Cucumis sativus未授粉胚珠诱导、油茶幼胚诱导和油茶未授粉子房诱导中体现[7, 26−28],证明TDZ在诱导愈伤组织方面有一定优势。本研究在NAA、TDZ和蔗糖的三因素三水平正交试验中发现,前二者对于诱导率的影响显著,而蔗糖不显著,此结果与靳皓然等[7]对油茶未授粉子房的研究是一致的,即细胞分裂素TDZ对诱导率的影响略大于NAA,因此适合油茶未授粉胚珠愈伤组织诱导的植物生长调节剂和蔗糖组合为:MS培养基+1.0 mg·L−1 NAA+2.0 mg·L−1 TDZ+40 g·L−1蔗糖。
本研究中的胚性愈伤组织质地紧密,呈浅绿色,表面有密集球状突起,此形态与五针松Pinus parviflora针叶胚性愈伤诱导[29]和‘凤丹白’Paeonia ostii ‘Phoenix White’[30]的成熟胚诱导中的胚性愈伤组织基本相同;而油茶未成熟幼胚诱导出的胚性愈伤组织则呈现黄褐色,结构疏松[2];荔枝Litchi chinensis的胚性愈伤组织黄白色,表面干燥,颗粒状明显[12];凤丹的胚性愈伤组织呈黄色团簇状,质地紧密[13]。胚性愈伤组织虽然在形态上有所不同,但从细胞学角度观察结果较为类似,细胞大小一致,排列紧密,细胞核大并易被染色,胚性愈伤组织会逐渐脱离原非胚性愈伤组织细胞而形成独立的几个细胞团,此时重新形成的胚性细胞团具有活跃的分生能力,是胚状体的前身[17]。且研究发现,胚性细胞团会出现极性,头部形成体积较小的规则胚性细胞,另一端则伸长为长条形胚柄细胞,两者共同组成原胚[31]。此现象在本研究的胚性愈伤组织中也有出现。
根据体视显微镜下的愈伤组织形态学观察结果,选取不同培养时间和不同形态的愈伤组织进行石蜡切片,从细胞学角度研究愈伤组织的起源及发育进程。愈伤组织有多种起源方式,可能从胚囊内部发生,也可能从外部的表皮细胞发生。外起源分为单细胞和多细胞起源2种[32]。本研究中油茶胚珠愈伤组织可能来源于珠被多细胞的分裂增殖,胚囊内部的细胞在愈伤组织诱导初期进行了旺盛的分裂活动,且形成胚性细胞团,但珠被细胞的增殖使得内部空间有限,胚性细胞团的发育受阻而停止,这与番茄Solanum lycopersicum未授粉胚珠的愈伤组织诱导中结果一致,其内部的愈伤组织在第6天时即停止生长[33],最终由珠被细胞诱导得到二倍体植株。珠被细胞继续进行分裂,形成结构疏松,排列不规则的非胚性细胞团。PERERA等[17]研究认为:此时珠被细胞进一步分裂增殖产生形成层状区,在这一区域会出现胚性愈伤组织和非胚性愈伤组织的转化,胚性细胞团逐渐脱离原细胞团形成分生结节,即多个分裂中心,是未分化胚状体的原始形态。本研究同样观察到表皮细胞分裂形成的非胚性细胞团逐渐出现独立的胚性细胞团,且向外凸出形成原胚,预测接下来可能会形成球形胚[2]。本研究从细胞学角度揭示了油茶未授粉胚珠产生愈伤组织的发育过程,外部表皮细胞增殖形成非胚性愈伤组织,后逐渐形成胚性愈伤组织,产生分生结节且有脱离母体组织的趋势,形成胚状体的原始形态。
流式细胞仪对愈伤组织的倍性检测结果显示:所获得的愈伤组织全部为六倍体,即体细胞产生的愈伤组织,结合上述的细胞学观察结果,基本可以确定愈伤组织来源于珠被细胞。韩春霞等[6]在油茶花药诱导愈伤组织的研究中获得了四倍体、六倍体、八倍体、十倍体和混倍体(三/六倍体)倍性的愈伤组织,推测可能是减数分裂异常或激素导致。在花药的愈伤组织诱导中可观察到花药壁破裂,小孢子细胞突破花药壁生长的现象[5],但在本研究中未能看到大孢子细胞突破胚珠壁细胞生长,可能是2层珠被细胞阻碍了内部大孢子细胞的生长发育,使得单倍细胞无法分裂增殖,所以本研究所得到的愈伤组织均来源于珠被体细胞。
-
油茶‘华金’优良品种的未授粉胚珠诱导愈伤组织的最适培养基配方为:MS培养基+1.0 mg·L−1 NAA+2.0 mg·L−1 TDZ+40 g·L−1蔗糖+7.0 g·L−1琼脂。研究结果显示:MS培养基能更好地满足胚珠愈伤组织诱导的需要,诱导率最高为72.50%;植物生长调节剂组合中1.0 mg·L−1NAA + 2.0 mg·L−1 TDZ使得愈伤组织诱导率最高,为88.89%;在正交试验中得到最佳培养基配方为:MS作为基础培养基,添加1.0 mg·L−1 NAA+2.0 mg·L−1 TDZ+40 g·L−1蔗糖。本研究得到1种胚性愈伤组织。质地紧密,外观呈浅绿色,表面有密集球状凸起;胚性细胞排列紧密,体积较小,细胞核易被染色,分裂能力强。愈伤组织发育过程为单倍体细胞最初进行胚性细胞的分生活动,但后期空间受限而停止分裂。珠被细胞进一步分裂,由非胚性转为胚性细胞,形成分生结节,并逐渐有脱离母体愈伤组织而独立的趋势,是为后期形成胚状体结构的原始形态。且愈伤组织的倍性鉴定结果为六倍体体细胞,因此愈伤组织细胞团起源于珠被细胞脱分化后的分裂增殖。
Callus induction and morphological and cytological observation of unpollinated ovules of Camellia oleifera
doi: 10.11833/j.issn.2095-0756.20220507
- Received Date: 2022-08-02
- Accepted Date: 2022-11-17
- Rev Recd Date: 2022-11-15
- Available Online: 2023-07-13
- Publish Date: 2023-08-20
-
Key words:
- Camellia oleifera /
- unpollinated ovule /
- callus /
- cytological characteristics /
- ploidy identification
Abstract:
| Citation: | JIN Haoran, YANG Shanwei, YUAN Leihuizi, et al. Callus induction and morphological and cytological observation of unpollinated ovules of Camellia oleifera[J]. Journal of Zhejiang A&F University, 2023, 40(4): 773-782. DOI: 10.11833/j.issn.2095-0756.20220507 |










 DownLoad:
DownLoad: