-
20世纪70年代以来,二氧化碳(CO2)浓度升高引起的气候变暖已成为广泛关注的热点问题[1]。土壤作为陆地最大碳库,储存着全球约三分之二的碳,并可通过土壤呼吸向大气排放占全球25%的碳[2]。土壤呼吸是由土壤微生物、植物根系、土壤动物共同作用产生CO2的生物生态学过程[2]。目前,土壤呼吸组分的研究集中于根系与微生物的贡献,而关于土壤动物对CO2排放贡献的研究却相对缺乏[3]。土壤动物直接释放的CO2约为土壤总呼吸的5%~10%,同时,土壤动物还会通过机械扰动、取食、筑巢及与微生物协同共生等间接方式调控CO2释放[4]。蚂蚁作为森林生态系统中种类和数量最丰富的土壤动物类群之一,不仅可通过自身代谢活动直接产生CO2,也可通过影响土壤的物理性质、化学转化和微生物代谢等调控土壤呼吸动态[5]。
已有研究表明:蚂蚁巢穴土壤中CO2的释放量显著高于非蚁巢土壤,这可能与蚂蚁取食和代谢活动积累大量有机物有关[6];蚁巢土壤内氮磷无机盐养分含量高于其周围土壤,从而增加土壤微生物量、活性及功能多样性,加速有机质分解产生CO2[7];同时,蚂蚁筑巢和代谢活动会增加巢穴中土壤含水率,而较高的湿度能够促进微生物分解有机物质,从而增加CO2的排放[8];另外,蚂蚁筑巢过程中对土壤的物理扰动以及其构建的大量通道改变了土壤质地和通气性,可能显著影响土壤CO2的排放[9]。因此,蚂蚁活动能够直接或者间接调控土壤呼吸动态。
近年来,国内外学者主要对寒带、温带、亚热带的森林、草地或农田土壤呼吸进行了研究,而关于热带森林土壤呼吸的研究却相对缺乏[10]。西双版纳地貌复杂、热量丰富、雨量充沛、干湿季分明,蚂蚁区系分布最为丰富多样[11]。本研究选择西双版纳小果野芭蕉Musa acuminata群落为样地,原位连续测定土壤呼吸速率的季节动态,分析蚂蚁活动引起土壤微生物及理化性质变化及其与土壤呼吸之间的关系,以期为精确评估热带雨林碳源汇过程提供基础数据,有助于探明蚂蚁活动对热带森林土壤呼吸动态影响的生物学机制。
-
研究区位于中国科学院西双版纳热带植物园(21°55′N,101°16′E)。该区属北热带季风气候,年均降水量为1 557 mm,年均气温为21.5 ℃。干湿季分明,5—10月为湿季,11月至翌年4月为干季。植物园中,常见的蚂蚁群落种类包括赛奇大头蚁Pheidole sagei、中华大头蚁Pheidole sinensis、棒刺大头蚁Pheidole spathifera、卡泼林大头蚁Pheidole capellini、宽结大头蚁Pheidole noda、横纹齿猛蚁Odontoponera transversa、爪哇厚结猛蚁Pachycondyla javana、毛发铺道蚁Teramorium ciliatum[12]。选择小果野芭蕉森林群落为研究样地,该样地海拔为535 m,群落植被以小果野芭蕉为主,此外还有董棕Caryota urens、勐仑翅子树Pterospermum menglungense等树种,盖度为85%;草本植物主要有刺通草Trevesia palmata、银叶砂仁Amomum sericeum等。土壤为砖红壤,凋落物层厚度为2~4 cm。
-
于2017年3、6、9及12月进行采样与测定,在样地中随机设置3个50 m×50 m重复样地,间隔约200 m。采用诱饵法寻找蚂蚁巢穴,发现样地中蚂蚁主要为大头蚁属Pheidole,在每个样地内选取6个大头蚁属蚁巢,宽度为(10.40±2.64) cm,深度为(9.50±1.80) cm,并设置6个距离巢穴5 m的非蚁巢地作为对照(共12个测点)。为了减少测定误差,土壤呼吸速率采用Li-6400-09呼吸仪,于8: 00—11: 00 测定,该时段土壤呼吸速率接近全天的平均值,代表性较好[13]。测定前将聚氯乙烯(PVC)圈(直径为10 cm,基本覆盖蚁巢)提前24 h插入测点,每个测点的测量(连续2次)约在5 min内完成,故每个样地的土壤呼吸测量约在1 h内完成(3 h完成3个样地的测定)。
分别采集0~5、5~10、10~15 cm共3层土样测定土壤性质;土壤温度(ST)、含水率(SW)、容重(BD)、pH分别采用便携式测量仪(SIN-TN8)、烘干称量法、环刀法和电位法测定;总有机碳(TOC)、易氧化碳(ROC)、微生物生物量碳(MBC)分别采用油浴加热-重铬酸钾容量法、高锰酸钾氧化法和氯仿熏蒸法测定;全氮(TN)、水解性氮(HN)、铵氮(
${\rm{NH}}^+_4$ -N)、硝氮(${\rm{NO}}^-_3$ -N)分别采用全自动凯氏定氮仪、碱解扩散法、氧化镁浸提扩散法和酚二磺酸比色法测定。 -
数据经整理后用Excel制表,用SPSS 23.0与Origin 2021软件统计分析。采用指数回归模型(Rs=aebTs)探究土壤呼吸速率与不同土层温度的关系,其中Rs为土壤呼吸速率(μmol·m−2·s−1),a、b、Ts分别为0 ℃时的呼吸速率、指数模型中温度反应系数、土壤温度(℃);Q10 (温度升高10 ℃土壤呼吸速率增加的倍数)采用指数模型(Q10=e10b)进行计算。采用Quadratic模型(Rs=ax2+bx+c)和Exponential模型(Rs=aebx)分析土壤呼吸速率与不同土层水分及微生物量碳之间的关系,a、b、c为回归系数。参考李霁航等[8]、赵吉霞等[14]及赵爽等[15]的方法,分别对不同土层温度、水分、微生物生物量碳与土壤呼吸做回归分析,比较其对土壤呼吸变化的解释程度。采用Pearson相关分析法分析小果野芭蕉热带森林群落的土壤理化性质相关性,主成分分析(PCA)法分析理化指标与土壤呼吸速率变化之间的相互关系。
-
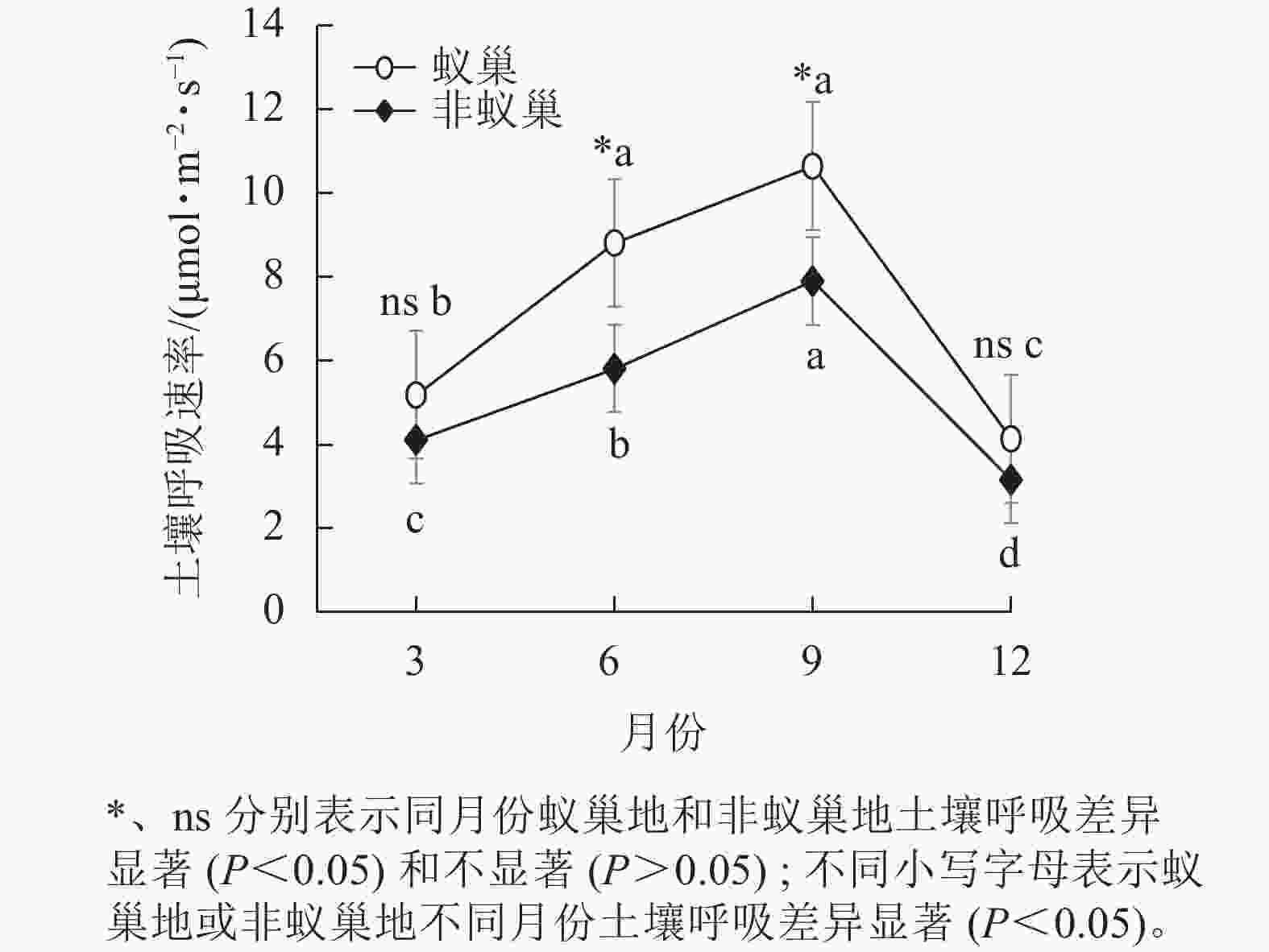
图1结果表明:4个月蚁巢平均土壤呼吸速率(7.19 μmol·m−2·s−1)显著高于非巢地(5.24 μmol·m−2·s−1),蚂蚁筑巢增加了37%的土壤呼吸速率。蚁巢地和非蚁巢地的土壤呼吸速率均随月份呈单峰型变化趋势,最大值出现在9月(10.64和7.90 μmol·m−2·s−1),最小值在12月(4.11和3.16 μmol·m−2·s−1),蚁巢地和非蚁巢地9月土壤呼吸速率分别为12月的2.57倍和2.50倍。蚁巢地和非蚁巢地土壤呼吸速率仅在6和9月差异显著(P<0.05)。
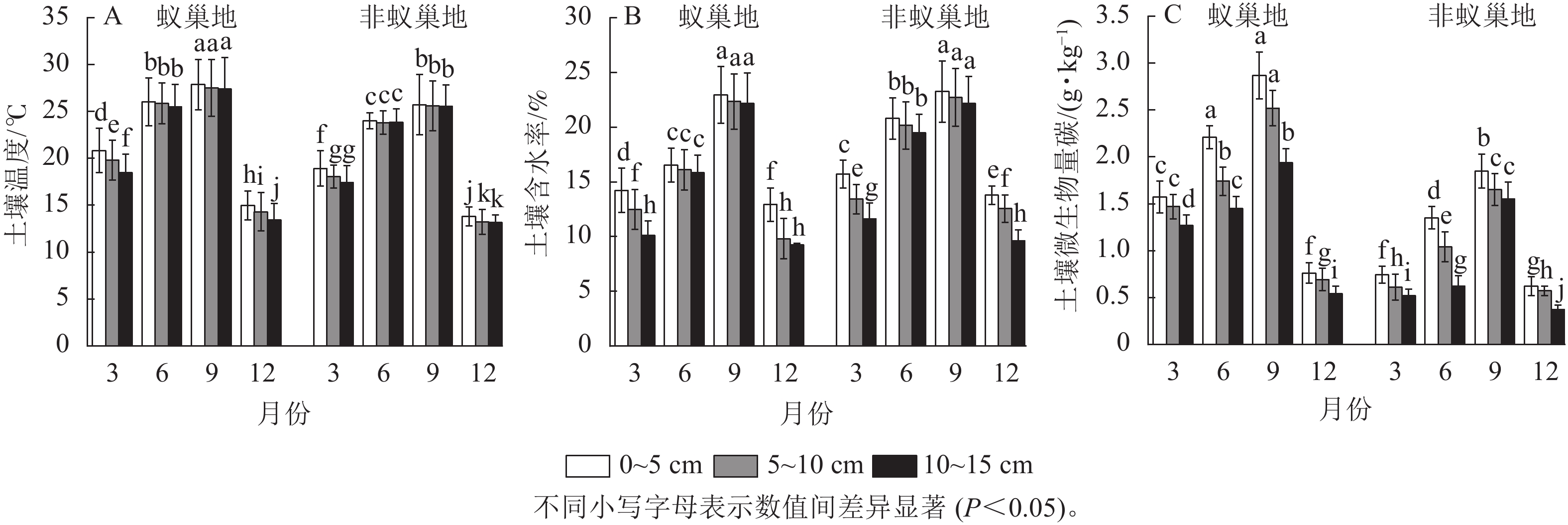
蚁巢地和非蚁巢地不同土壤温度、土壤含水率及微生物生物量碳质量分数均呈现与土壤呼吸速率相似的单峰型季节变化,其中最大值在9月(图2)。不同月份蚁巢地3个土层温度与微生物生物量碳质量分数均显著高于非蚁巢地(P<0.05),3、6、9和12月蚁巢3个土层平均温度增加量分别为8.72%、8.03%、7.76%和6.22%,而微生物生物量碳则分别提升了130.00%、78.81%、44.87%和28.36%。除9月外,蚁巢地不同土层含水率显著低于非蚁巢地(P<0.05),3、6和12月3个土层平均值分别降低了10.73%、24.74%和12.59%。结果表明蚂蚁筑巢影响土壤温湿度及微生物生物量碳的季节变化。
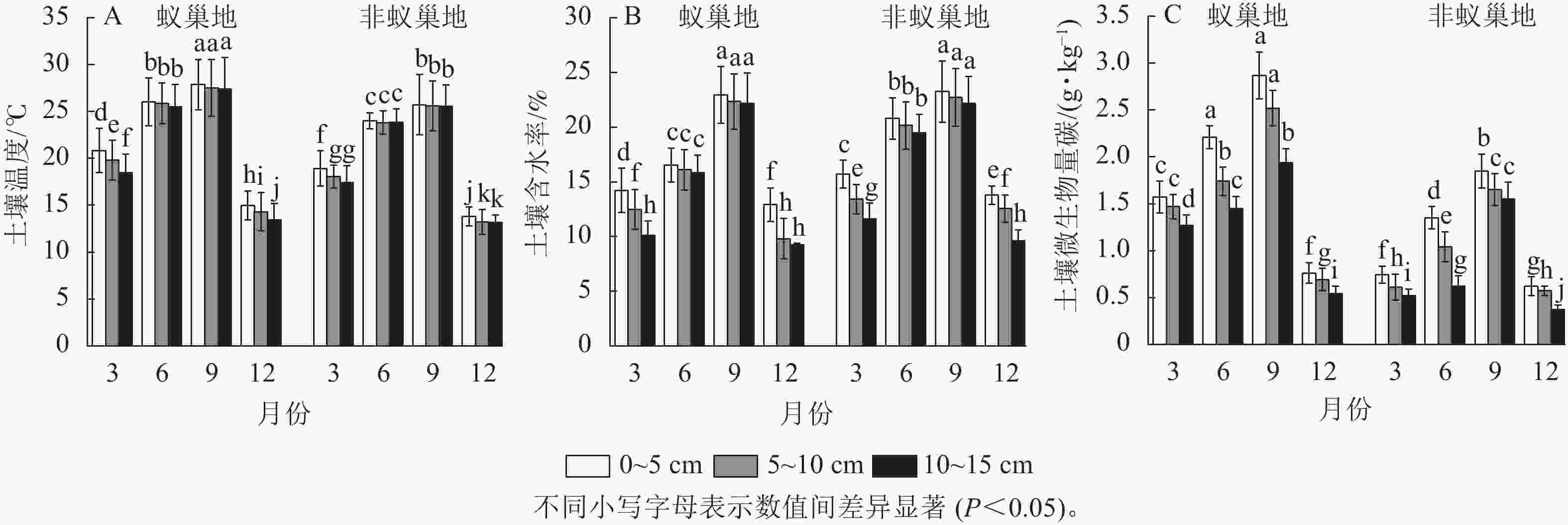
Figure 2. Effects of ant nesting on spatiotemporal dynamics of temperature, water, and microbial biomass carbon in soils
蚁巢地与非蚁巢地不同月份土壤温度、土壤含水率及微生物生物量碳质量分数均表现为随着土层的加深而下降。相较于0~5 cm土层,10~15 cm蚁巢地平均土壤温度、土壤含水率和微生物生物量碳质量分数分别下降了5.53%、13.87%、29.73%,而非蚁巢地10~15 cm土层平均土壤温度、土壤含水率和微生物生物量碳质量分数较0~5 cm土层分别下降了3.10%、14.46%、32.46%。结果表明蚂蚁筑巢显著改变了土壤温湿度及微生物生物量碳沿土层的垂直分布特征。
-
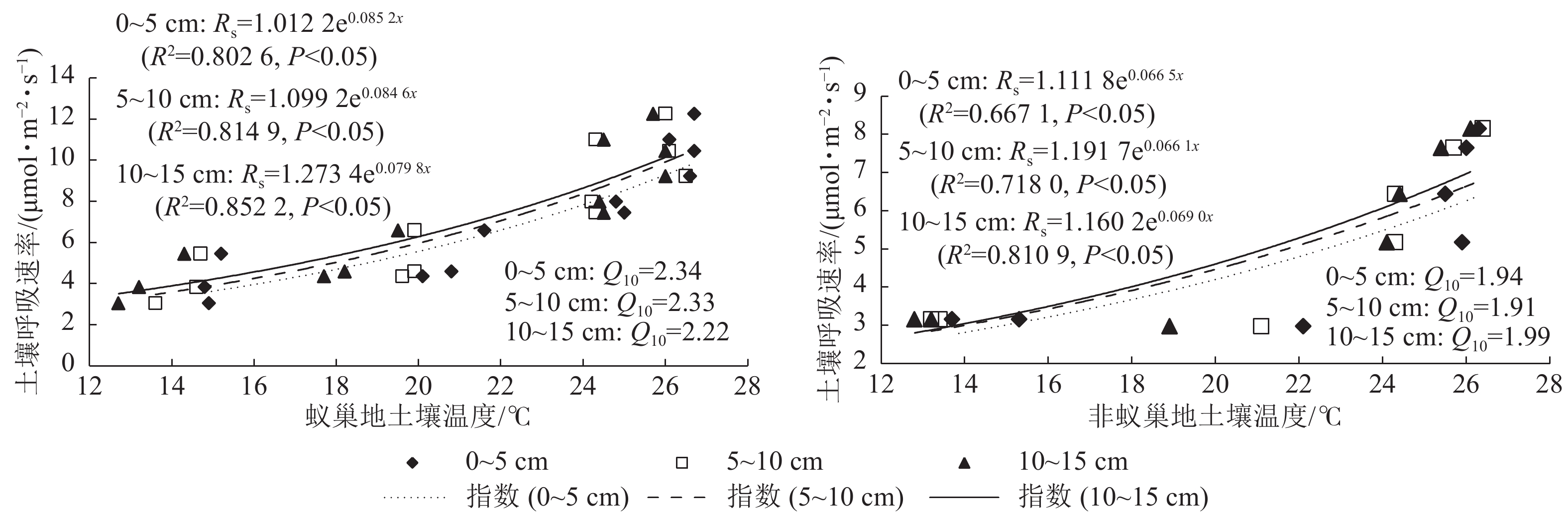
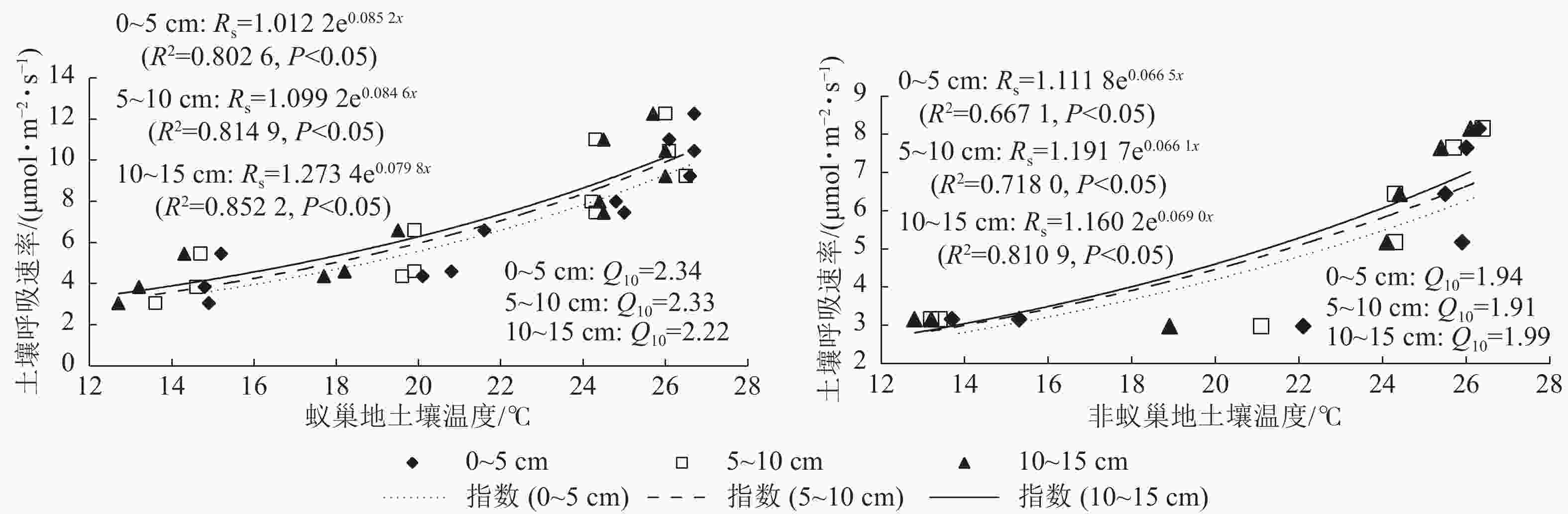
蚁巢地与非蚁巢地土壤呼吸速率均随不同土层温度呈指数增加的变化趋势(图3,P<0.05)。不同土层温度变化对土壤呼吸速率的解释率蚁巢地(80.26%~85.22%)均高于非蚁巢地(66.70%~81.09%);蚁巢地与非蚁巢地不同土层温度对土壤呼吸速率变化的解释率从低到高依次为0~5、5~10、10~15 cm。不同土层土壤呼吸速率对温度的敏感性(Q10)为蚁巢地(2.22~2.34)高于非蚁巢地(1.91~1.99)。蚁巢地土壤Q10最大值出现在0~5 cm土层,而非蚁巢地土壤Q10最大值则在10~15 cm土层。可见,蚂蚁筑巢引起的土层温度变化对土壤呼吸速率产生了重要影响。
蚁巢地与非蚁巢地土壤呼吸速率均随不同土层水分呈二次增加趋势(表1, P<0.01)。不同土层水分变化对蚁巢地土壤呼吸速率的解释率蚁巢地(71.96%~79.01%)均小于非蚁巢地(76.45%~89.28%)。蚁巢地与非蚁巢地不同土层水分对土壤呼吸速率的解释率均表现为上层土壤低于下层土壤。
处理 土层/cm 方程 R2 P 蚁巢地土壤 0~5 Rs=−0.021 9x2 + 1.386 1x−9.361 6 0.719 6 0.001 5~10 Rs=−0.018 6x2 + 1.247 2x−7.126 9 0.754 9 0.001 10~15 Rs=−0.030 7x2 + 1.626 5x−8.939 7 0.790 1 0.002 非蚁巢地土壤 0~5 Rs=−0.007 2x2 + 0.683 5x−4.679 9 0.764 5 0.000 5~10 Rs=−0.000 5x2 + 0.367 7x−0.940 6 0.8753 0.001 10~15 Rs=−0.000 6x2 + 0.553 0x−1.125 6 0.8928 0.002 Table 1. Linkage of soil respiration rate (Rs) with soil water content (x) in different soil layers
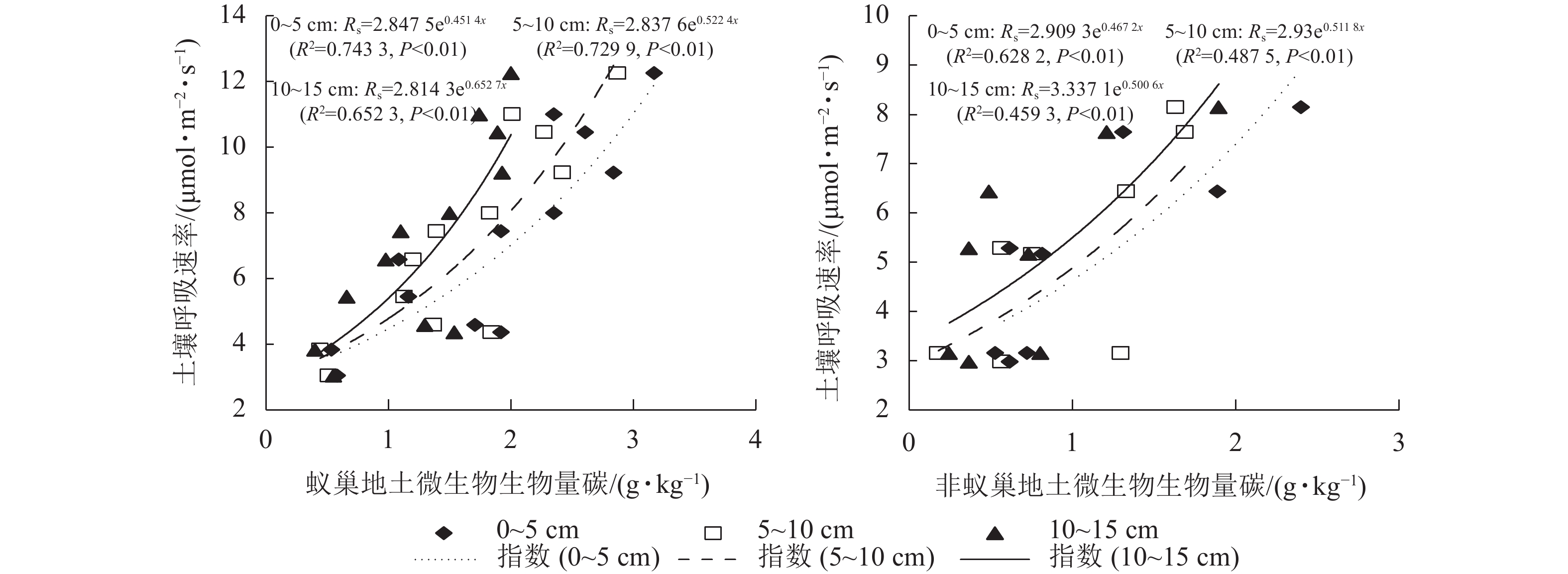
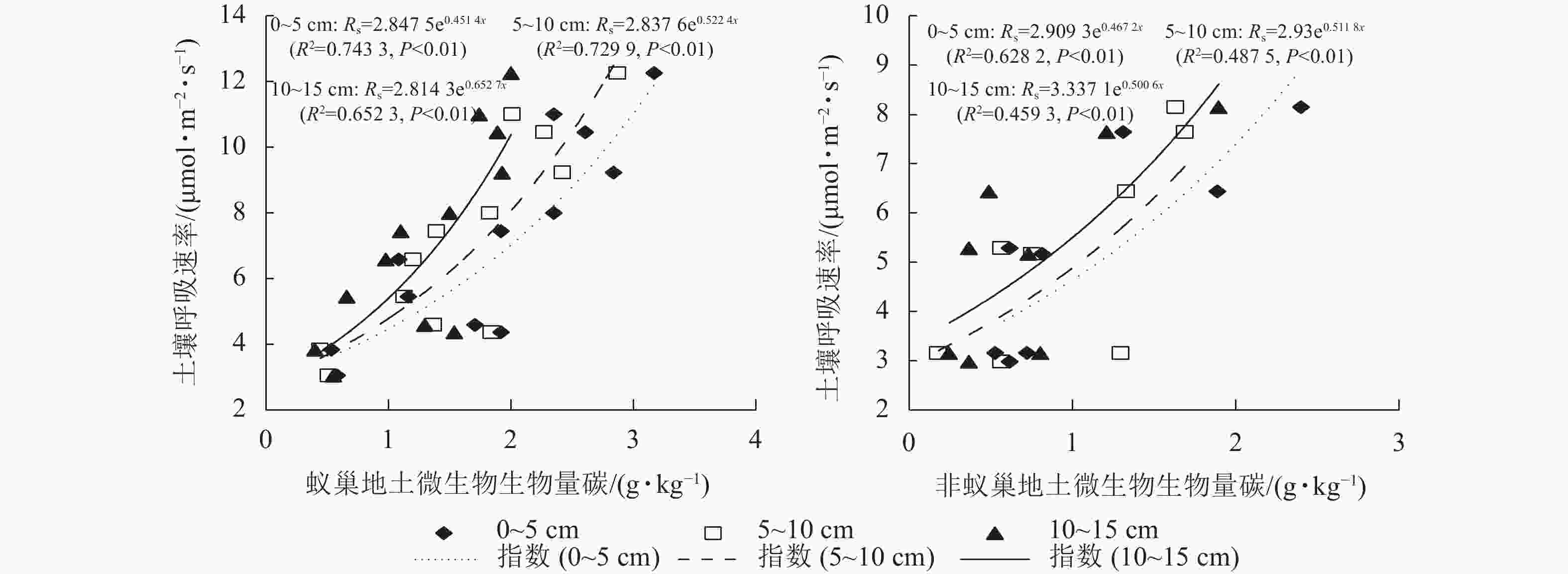
蚁巢地与非蚁巢地土壤呼吸速率均随不同土层微生物生物量碳质量分数呈指数增加的变化趋势(图4,P<0.01)。不同土层微生物生物量碳质量分数变化对土壤呼吸速率的解释率表现为蚁巢地(65.23%~74.33%)均高于非蚁巢地(45.93%~62.82%)。蚁巢地与非蚁巢地不同土层微生物生物量碳质量分数对土壤呼吸速率的解释率从高到低依次为0~5、5~10、10~15 cm。
-
表2结果表明:蚂蚁活动显著影响了土壤理化性质(P<0.05)。相较于非蚁巢地,蚁巢地土壤总有机碳、易氧化有机碳和微生物生物量碳质量分数分别增加了5.29%、31.98%、27.20%;全氮、水解氮、硝态氮和铵态氮质量分数分别提升了40.00%、39.81%、17.41%、65.95%;土壤pH上升了19.20%,而容重则降低了27.45%。
处理 容重/
(g·cm−3)pH 总有机碳/
(g·kg−1)易氧化碳/
(g·kg−1)微生物生物量碳/
(g·kg−1)全氮/
(g·kg−1)水解氮/
(mg·kg−1)硝态氮/
(mg·kg−1)铵态氮/
(mg·kg−1)蚁巢地 1.02±0.09 b 6.83±0.31 a 35.22±6.00 a 16.55±3.91 a 1.59±0.75 a 0.91±0.80 a 213.42±57.38 a 2.90±1.55 a 16.96±5.48 a 非蚁巢地 1.30±0.16 a 5.73±0.25 b 33.45±7.60 b 12.54±3.77 b 1.25±1.97 b 0.65±0.39 b 152.65±94.42 b 2.47±1.05 b 10.22±6.24 b 说明:不同小写字母表示蚁巢地和非蚁巢地土壤理化性质差异显著(P<0.05)。 Table 2. Effects of ant nesting on soil physicochemical properties
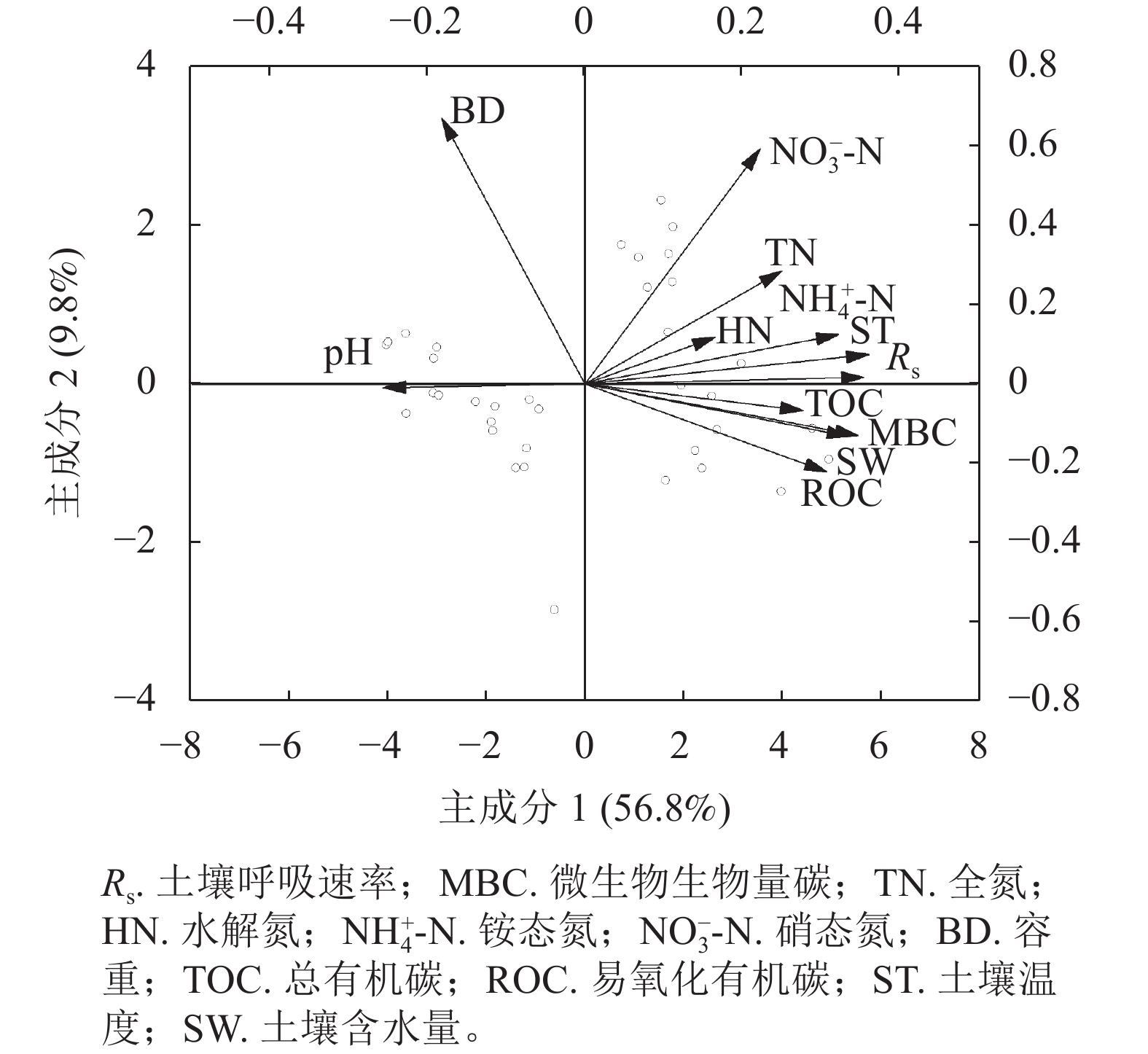
图5结果表明:主成分1和主成分2对土壤呼吸的贡献率分别为56.8%和9.8%,累计解释了66.6%的数据变异。按箭头夹角来看,温度、水分、微生物生物量碳、总有机碳、铵态氮与土壤呼吸速率的夹角较小,说明它们是调控土壤呼吸速率变化的主控因子,全氮、水解氮和易氧化有机碳对土壤呼吸的贡献率次之,而硝态氮的贡献最小。另外,容重和pH与呼吸速率的夹角为钝角,与土壤呼吸速率呈负相关性且贡献亦较少。
-
本研究显示:无论是高温还是低温季节,蚁巢地土壤呼吸速率均显著高于非蚁巢地。在高温季节,蚂蚁的筑巢觅食等活动显著增加,在巢穴富集大量有机物质,促进了微生物的活动,使得土壤呼吸增强。降温时,蚁巢缩小气孔导致空气对流减少,从而稳定巢内温度,维持了土壤呼吸的稳定[16]。蚁巢地土壤呼吸季节变化最大值出现在9月,也与蚂蚁季节性生物活性相吻合。如红褐林蚁Formica rufa巢穴排放CO2速率在5—9月显著高于10月至翌年4月,这是因为红褐林蚁在5—9月的活跃程度更高[17]。因此,蚂蚁季节性的活动能够影响土壤呼吸速率的时间变化。
-
回归分析与主成分分析结果表明:较高的巢温对土壤呼吸速率变化具有较大的贡献。这可能与蚂蚁筑巢活动调节巢内温度有关,蚂蚁将土壤有机质微粒搬运至地下建造巢穴具有更强的隔热作用,有助于保持蚁巢的保温作用[18]。同时,较高土温有利于微生物的活动,加速有机物分解过程中的碳排放[19]。土壤温度处于6~35 ℃范围内,升高温度能提升土壤酶与底物的亲和力[20],从而刺激土壤微生物的碳矿化过程。因此,蚂蚁筑巢活动导致土壤温度变化是土壤呼吸升高的一个重要驱动因素。
蚁巢地土壤水分显著低于非蚁巢地。可能是因为在降雨时,蚂蚁会将巢穴入口封闭,从而减少雨水渗入。也可能由于蚂蚁巢穴的孔隙度大,土壤持水能力弱,水分快速渗入更深的土层,使得巢穴水分减少[16]。但也有研究表明蚂蚁取食活动及生理代谢会增加穴内水分含量[21]。蚂蚁的筑巢行为可能导致土壤孔隙度增加,使得雨水更容易渗入蚂蚁巢穴中,提高土壤含水率[22]。
蚁巢地土壤水分变化显著影响土壤呼吸。一般情况下,土壤含水率较低时,水分的增加能够刺激土壤呼吸速率[23]。土壤含水率过高时,好氧微生物和植物根系因缺氧而导致活性下降,土壤呼吸强度则会降低[24-25]。含水率高的非蚁巢地土壤呼吸速率小于低含水率的巢穴,可能是本研究地降雨丰富,土壤湿润,水分对土壤呼吸的影响比其他理化因子的影响相对较小。
-
蚁巢地土壤微生物量碳质量分数显著高于非蚁巢地,对土壤呼吸速率的解释量可达65.23%~74.33%。说明蚁巢地更高的微生物数量对土壤呼吸速率产生了重大影响。这可能是由于蚂蚁取食活动在巢穴中积累动植物残体,代谢活动产生大量分泌物,富集了充足的营养元素,为微生物提供了代谢底物及养分;蚂蚁筑巢建穴活动使得巢穴中温度较高、湿度适宜,为微生物增殖提供了适宜的微环境,使蚂蚁巢穴中微生物数量显著高于非蚁巢地[21]。有研究发现:蚂蚁巢穴中较高的有机质和养分含量,使巢穴中细菌和真菌的数量比非巢地土壤高10倍以上,且促进了巢穴周围植物根系的生长和代谢活动[26]。蚂蚁巢穴较高的氮素养分含量有利于周围植物根系的生长,同时促使植物根系产生大量分泌物,使得蚂蚁巢穴中有机物积累更多,从而使得微生物数量增加[27]。
土壤微生物生物量碳质量分数变化能刺激微生物代谢活动,从而显著影响土壤呼吸。何甜甜等[28]研究表明:土壤呼吸速率与微生物生物量碳质量分数呈显著正相关。王泽西等[29]研究发现:对土壤施加氮肥能增加土壤微生物生物量碳质量分数,从而显著促进土壤微生物的呼吸。于舒等[30]发现在土壤温湿度适宜时,土壤微生物量碳质量分数的增高能够加速有机碳矿化过程CO2释放。因此,蚂蚁活动能通过介导土壤微生物数量而调控土壤呼吸过程与动态。
-
巢穴总有机碳、易氧化有机碳、硝态氮及铵态氮等养分质量分数显著高于非蚁巢地。可能是蚂蚁采集植物残体昆虫尸体等作为食物带入巢穴,以及食物残渣和排泄物质在巢中积累,大量增加了巢穴中土壤的碳氮含量[31]。蚂蚁也可以影响巢穴内植物根系的分泌物,使有机物和矿物质颗粒包裹在一起,提高有机质稳定性[16]。蚂蚁巢穴内碳氮有机物含量高,促进有机氮矿化速率,进而导致蚁巢土壤大量的硝酸根离子和铵根离子积累,碳氮释放可能对土壤呼吸速率产生重大影响[32]。有研究认为:土壤高碳氮含量能够提高土壤酶活性、微生物丰富度及代谢速率,从而促进土壤呼吸[33]。土壤有机碳能为土壤微生物呼吸提供代谢底物,而土壤无机氮则能促进植物根系呼吸,刺激土壤微生物的分解代谢[34],进而影响土壤呼吸。因此,蚂蚁活动能提高土壤碳氮含量,促进土壤呼吸。
-
土壤呼吸速率与pH和容重呈现显著的负相关。这可能是由于蚂蚁挖掘土壤、搬运食物等一系列活动显著降低了土壤容重,增加了土壤通气性,从而刺激了土壤呼吸速率[35]。鱼小军等[36]发现蚁巢地土壤容重比非蚁巢地低59%。蚂蚁建造巢穴时造成土壤颗粒分离,使土壤的孔隙度增大,且蚂蚁筑巢时会选择有利于保持通道稳定的细颗粒,使土壤容重降低[37]。同时,土壤容重的改变会影响土壤透气性、水分、温度等因子,进而影响土壤呼吸[21]。有研究表明:蚂蚁取食、代谢活动会使得蚂蚁巢穴土壤富集有机酸,导致土壤pH降低[38]。但也有研究表明:蚂蚁巢穴内有机物质积累及温度水分变化,可导致氮磷养分溶解度增加,最终使得土壤pH偏向中性[31]。微生物在一定的弱酸土壤环境中,代谢活性强,增长迅速,呼吸强度高。因此,蚂蚁活动引起土壤容重和pH改变,可能对土壤呼吸产生间接调控作用。
-
蚂蚁活动显著刺激了土壤呼吸速率,相较于非蚁巢地其增幅高达37%。蚁巢地土壤温湿度、微生物生物量碳质量分数对土壤呼吸季节变化的解释率高达65.23%~85.22%。蚂蚁筑巢增加土壤温度、微生物生物量碳、总有机碳、铵态氮、却降低土壤水分,其变幅为5.29%~65.95%;主成分分析表明它们是调控土壤呼吸变化的主控因子。因此,蚂蚁筑巢主要通过影响土壤微气候(温湿度)及碳氮养分含量而驱动蚁巢土壤的CO2排放。
Effects of ant activities on seasonal dynamics of soil respiration in the Musa acuminata communities of Xishuangbanna
doi: 10.11833/j.issn.2095-0756.20220533
- Received Date: 2022-08-17
- Accepted Date: 2023-01-05
- Rev Recd Date: 2022-10-08
- Publish Date: 2023-05-20
-
Key words:
- ant nest /
- Musa acuminata /
- tropical forests /
- soil respiration /
- seasonal fluctuation
Abstract:
| Citation: | FAN Yuxiang, YANG Bo, LI Yanmei, et al. Effects of ant activities on seasonal dynamics of soil respiration in the Musa acuminata communities of Xishuangbanna[J]. Journal of Zhejiang A&F University, 2023, 40(3): 502-510. DOI: 10.11833/j.issn.2095-0756.20220533 |













 DownLoad:
DownLoad: