-
氧化亚氮(N2O)是三大温室气体之一,在100 a尺度下,其增温潜势是二氧化碳(CO2)的298倍[1],同时,N2O还会破坏大气臭氧层[2]。有研究显示:现在大气中N2O浓度与工业革命前相比提高了20%,且仍以每年0.25%的速率在增加[3]。农田土壤是大气N2O的主要来源之一,人为排放的N2O约42%来自农业土壤[4]。其中,稻田土壤N2O排放量约占农田总排放量的22%,是大气N2O的一个重要排放源[5]。因此,减少稻田土壤中N2O的排放对保护环境及缓解全球变暖具有重要意义。
硝化作用是全球氮循环的关键过程,是土壤N2O产生的主要途径之一[6]。氨氧化是硝化作用的第1步,也是其限速步骤,主要由氨氧化细菌(ammonia-oxidizing bacteria,AOB)和氨氧化古菌(ammonia-oxidizing archaea,AOA)参与完成[7]。有研究表明:AOA在酸性、低NH4 + 含量的土壤中对硝化作用起主要作用,而AOB在中性和碱性土壤中起主导作用[7]。施用氮肥会促进氨氧化微生物的增长,从而增加N2O的排放[8]。因此,抑制氨氧化微生物的丰度和活性,可以降低土壤硝态氮的累积速率,抑制硝化作用,减少N2O排放[9]。硝化抑制剂是人们为了减少氮素损失、提高氮肥利用率而开发的一类化学或天然生物制剂;它对硝化细菌具有一定的毒性,可以抑制某些硝化细菌的丰度和活性,抑制土壤硝化作用的发生强度[10]。2-氯-6-三氯甲基吡啶(CP)和3,4-二甲基吡唑磷酸盐(DMPP)是2种农业生产上常用的硝化抑制剂,具有少量高效、作用时间长、对环境友好等优点[11]。杨秀霞等[12]通过大田试验表明:氮肥配施CP不仅可以增加水稻Oryza sativa产量17.6%~26.5%,还可以显著提高水稻对氮肥的吸收利用水平。在田间微区试验中,施用CP可以减少氮素损失21.7%~28.1%[13]。李杰等[5]通过室内培养实验发现:DMPP可以有效抑制AOB生长,减少水稻土中N2O排放。硝化抑制剂的抑制效果与土壤pH、有机质、含水量等环境因素有着密切的关系[14]。MENÉNDEZ等[15]研究发现:土壤含水量和温度是影响硝化抑制剂效果的关键因子,在低温潮湿的环境中DMPP可以更有效地减少N2O排放。PUTTANA等[16]研究表明:硝化抑制剂的抑制效率随着土壤pH的升高而逐渐降低。
目前,前人的研究多是关注硝化抑制剂在旱地土壤上的作用效果[17],也有学者研究硝化抑制剂对稻田土壤氮转化的影响[18]。但对于DMPP和CP这 2种硝化抑制剂在稻田土上的应用效果,尤其是对N2O排放影响的相关研究较少。因此,本研究以浙江省诸暨地区稻田土为对象,使用DMPP和CP进行室内培养试验,比较不同硝化抑制剂对稻田土硝化作用的抑制效果,以及其对N2O排放和氨氧化微生物的影响,以期为硝化抑制剂在稻田土上的应用提供科学依据。
-
供试稻田土采自浙江省诸暨市暨南街道(29°43′N,120°14′E),采集自0~20 cm耕层土壤,剔除杂物及残留根系,风干后过2 mm筛备用。土壤的基本理化性质如下:pH 7.17,有机质27.49 g·kg−1,全氮1.59 g·kg−1,碱解氮306.18 mg·kg−1,有效磷41.33 mg·kg−1,速效钾197.35 mg·kg−1。
-
共设4个处理,分别为:①不添加尿素和硝化抑制剂(ck,空白对照);②尿素[添加量为氮(N) 200 mg·kg−1,U];③尿素+CP (添加量为氮量的2%,CP);④尿素+DMPP (添加量为氮量的1%,DMPP)。称取20 g风干土置入100 mL玻璃瓶中,将土壤含水量调节至田间持水量的40%,在25 ℃培养箱中预培养5 d,以激活微生物。预培养后,按要求加入相应的蒸馏水、尿素和(或)硝化抑制剂,同时将土壤含水量调至田间持水量的60%,将4个处理组随机分布在25 ℃的恒温培养箱中。每隔1 d随机排序以减少温度的干扰。隔4 d补充1次水分,以保持土壤含水量恒定。在培养开始后的0、7、14、21 d进行破坏性取样,每个处理取3个重复样品,用于测定土壤pH、无机氮质量分数和氨氧化微生物丰度。
-
土壤pH采用pH计进行测定(土水质量比为1.0∶2.5)。铵态氮(NH4 + -N)和硝态氮(NO3 − -N)测定:称取10 g土样,加入50 mL 2 mol·L−1 氯化钾溶液,然后用AA3型连续流动分析仪测定NH4 + -N和NO3 − -N质量分数。N2O采集与测定:在加入尿素的1、2、5、7、10、20、21 d采集气体样品,采样前,用真空泵将橡胶塞封闭玻璃瓶中的空气抽空,5 h后用10 mL针筒采集瓶内气体,然后用GS-2014气象色谱仪进行N2O分析。N2O排放速率(F,ng·kg−1·h−1)公式:$F=\mathrm{\rho }\times \mathrm{\Delta }{C}/\mathrm{\Delta }{t}\times 273.15/ (273.15+T)\times V/m$。其中,ρ为标准状态下N2O的密度,为1.25 kg·m−3;ΔC/Δt为玻璃瓶内N2O浓度变化率(109·h−1);V为玻璃瓶上部有效空间体积(m3);T为环境气温(℃);m为土壤的干质量(kg)。土壤N2O累积排放量(M,μg·kg−1)公式:$M={ {F}}_{1}\times 24+\displaystyle \sum\limits _{i=2}^{n}\left({F}_{i}+{F}_{i-1}\right)/ 2\times \left({t}_{i}-{t}_{i-1}\right)\times 24$。其中,F为N2O排放速率(μg·kg−1·h−1);t为采样时间(d);i为采样次数;ti‒ti‒1为2次采样的间隔时间(d)。
-
采用Fast DNA SPIN试剂盒(MP Biomedicals)提取土壤总DNA,具体操作步骤按照说明书进行。将提取的土壤总DNA分管保存于−20 ℃冰箱中备用。实时荧光定量PCR(RT-qPCR)在CFX96 Optical Real-Time Detection System(Bio-Rad Laboratories)上进行检测。AOA和AOB的引物见表1。反应体系为20.0 μL:包括DNA模板2.0 μL,Green Premix Ex Taq 10 μL,正反引物各0.8 μL以及每管6.4 μL灭菌超纯水。AOA和AOB的反应条件均为:94 ℃下初始变性5 min,94 ℃下变性10 s,55 ℃变性15 s,72 ℃延伸45 s,40个循环。阴性对照采用灭菌超纯水作为模板。每个样品重复3次。根据扩增循环阈值(CT)、已知质粒浓度和阿伏伽德罗常数计算AOA、AOB amoA基因拷贝数。
-
净硝化速率(n)的计算公式:$ n=({{n}_{1}-{n}_{2}})/{t} $。其中,t为培养天数,n1为土壤样品NO3 − -N初始质量分数,n2为培养第t天时土样NO3 −-N质量分数。
数据采用Excel 2007和Origin 2019b进行统计分析和作图,采用SPSS 13.0进行方差分析、显著性检验(0.05水平)及相关性分析。
-
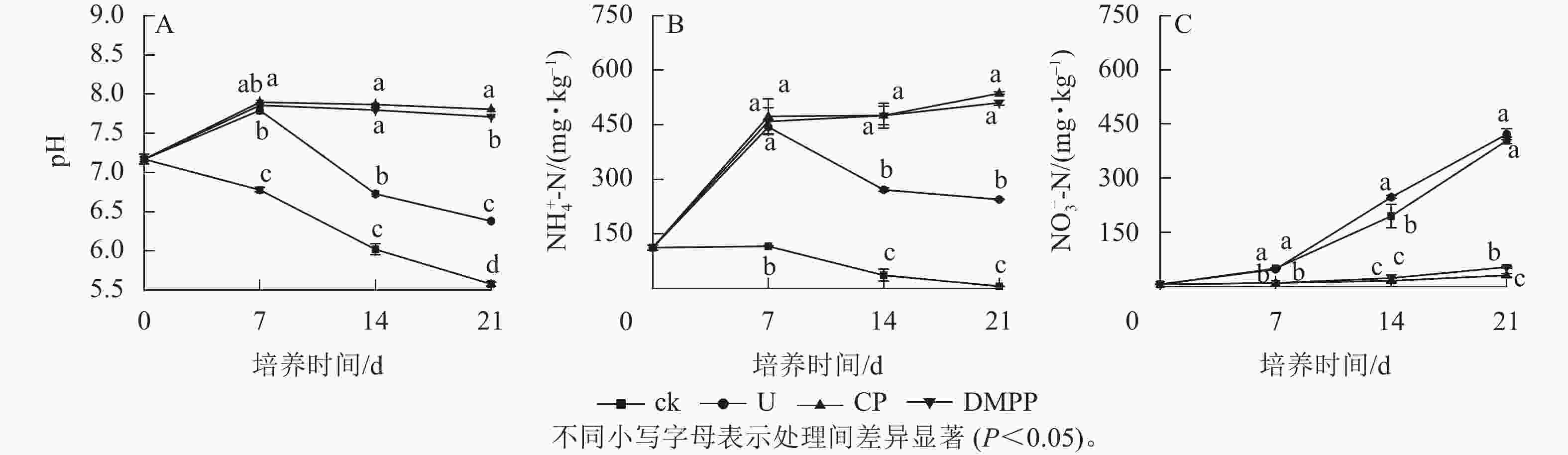
如图1A所示:在整个培养期间,ck处理的pH都呈下降的趋势。与ck相比,尿素的施用显著提高了土壤pH (P<0.05)。在0~7 d,U、CP和DMPP处理的pH均呈上升趋势,并在第7天达到最高。7 d后,U处理的pH快速下降直至培养结束;而DMPP与CP处理的pH在7 d后保持稳定。21 d时,DMPP与CP处理的pH分别为7.71和7.81,与U处理的pH 6.38相比,分别提高了20.8%和22.4%。
-
由图1B可以看出:U处理的土壤NH4 + -N质量分数呈先上升后下降的趋势。在前7 d,添加尿素处理的NH4 + -N质量分数迅速增加,达458.7 mg·kg−1,显著高于ck处理的112.2 mg·kg−1(P<0.05),表明尿素的添加显著提高了土壤NH4 + -N质量分数。而在7 d后,U处理的NH4 + -N质量分数快速下降,到21 d时降为244.5 mg·kg−1。硝化抑制剂处理的NH4 + -N质量分数在7 d后保持在一个稳定的状态,在14 d后还有小幅度上升。到21 d时,CP与DMPP处理的NH4 + -N质量分数分别为536.4和510.4 mg·kg−1,显著高于U处理(P<0.05),表明DMPP与CP显著抑制氨氧化作用。如图1C所示:各处理的NO3 − -N质量分数均随着培养时间的延长而逐渐上升。ck与U处理的NO3 − -N质量分数在前7 d上升缓慢,在7 d后快速上升,直到21 d。DMPP和CP处理的NO3 − -N质量分数在前14 d内基本保持不变,在14 d后略有上升。DMPP与CP处理的NO3 − -N质量分数显著低于ck和U处理(P<0.05)。
如表2所示:与ck和U处理相比,硝化抑制剂处理(DMPP与CP)显著降低了土壤净硝化速率(P<0.05)。而U与ck处理的净硝化速率之间没有显著差别。
间隔时间/d 净硝化速率/(mg·kg−1·d−1) ck U CP DMPP 1~7 6.30±0.24 a 5.99±0.06 a 0.56±0.09 b 0.55±0.09 b 7~14 20.71±4.91 b 28.47±0.66 a 0.82±0.73 c 1.93±1.35 c 14~21 29.83±5.56 a 24.89±1.56 a 2.18±1.24 b 4.33±1.38 b 说明:数值为平均值±标准误。同行不同小写字母表示处理间差异显著(P<0.05)。 Table 2. Net nitrification rate of each treatment for the three incubation intervals
-
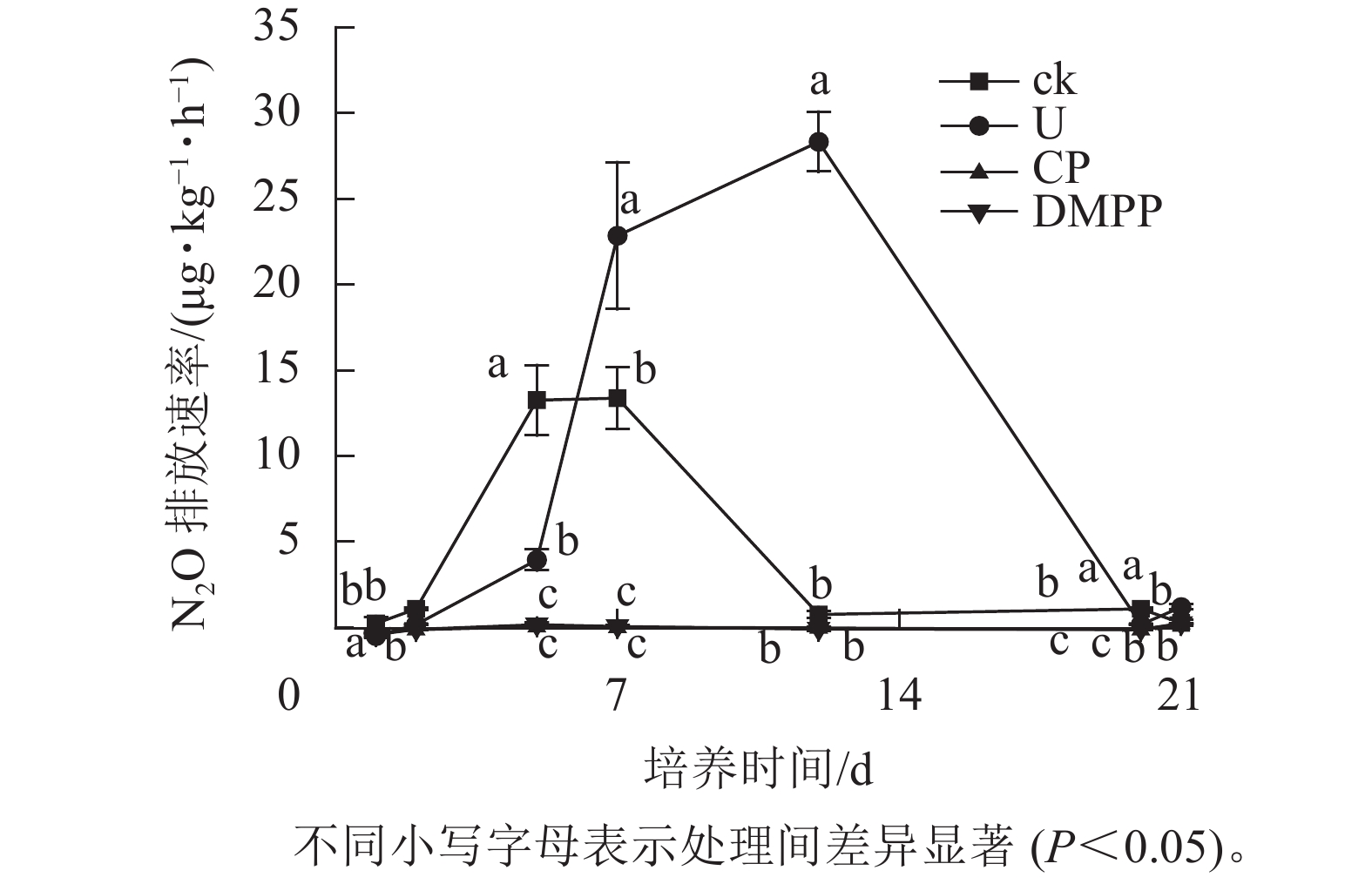
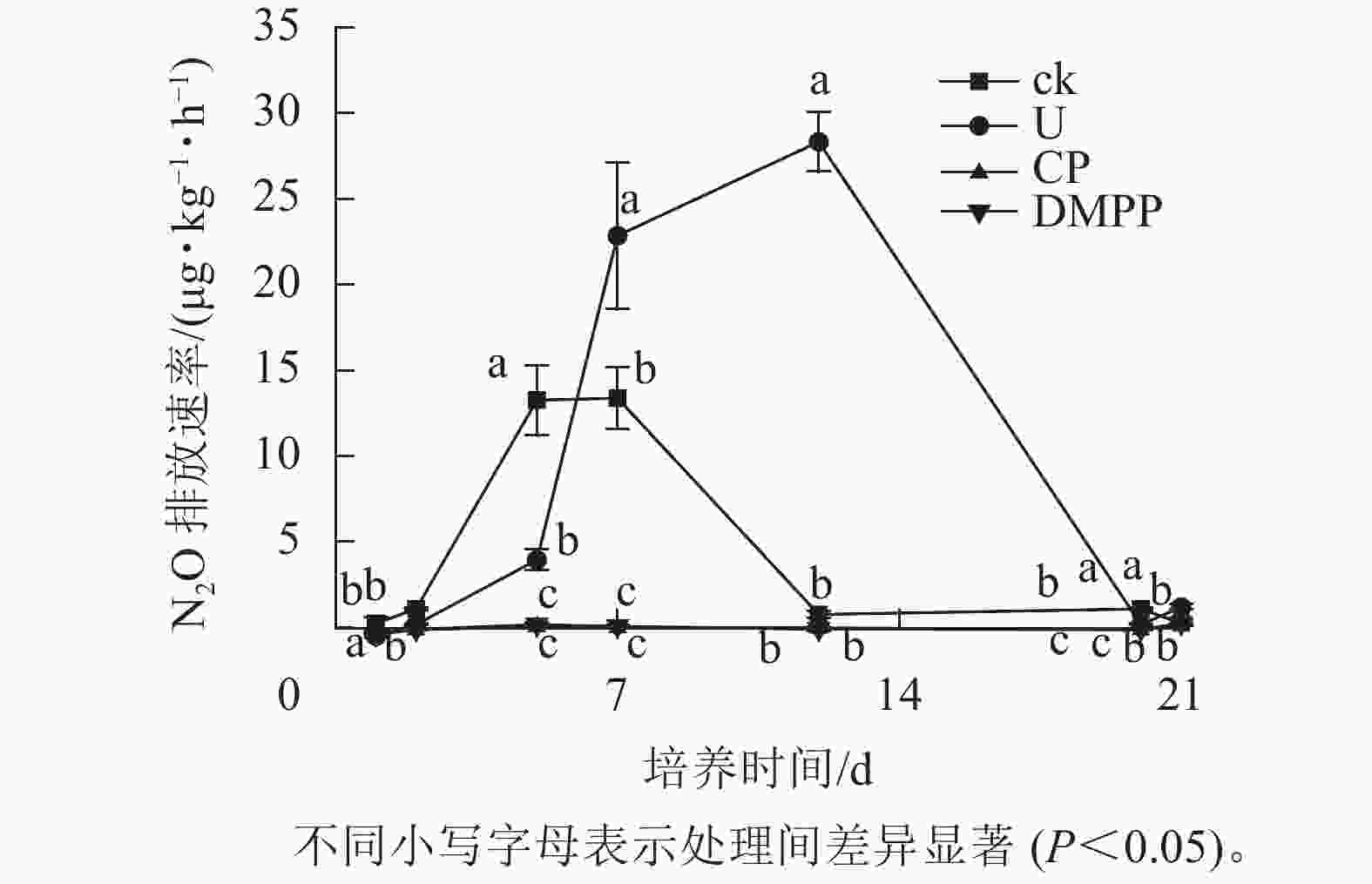
如图2所示:ck处理的2个排放峰分别出现在培养5和7 d,在到达第2个排放峰后,N2O的排放速率开始快速下降,到12 d时到达较低水平并持续到培养结束。与ck处理相比,U处理的排放峰出现较晚,分别出现在7 与12 d,且在培养7 d后,U处理的N2O排放速率远高于其他处理,直到培养结束时N2O的排放速率才降低到和其他处理相近的水平。这表明尿素的添加推迟了土壤N2O排放峰值的出现,提高了N2O的排放速率,同时延长了N2O排放的时间。与处理U相比,DMPP与CP处理的N2O排放速率在整个培养期间均处在较低水平,且两者之间没有显著差异。
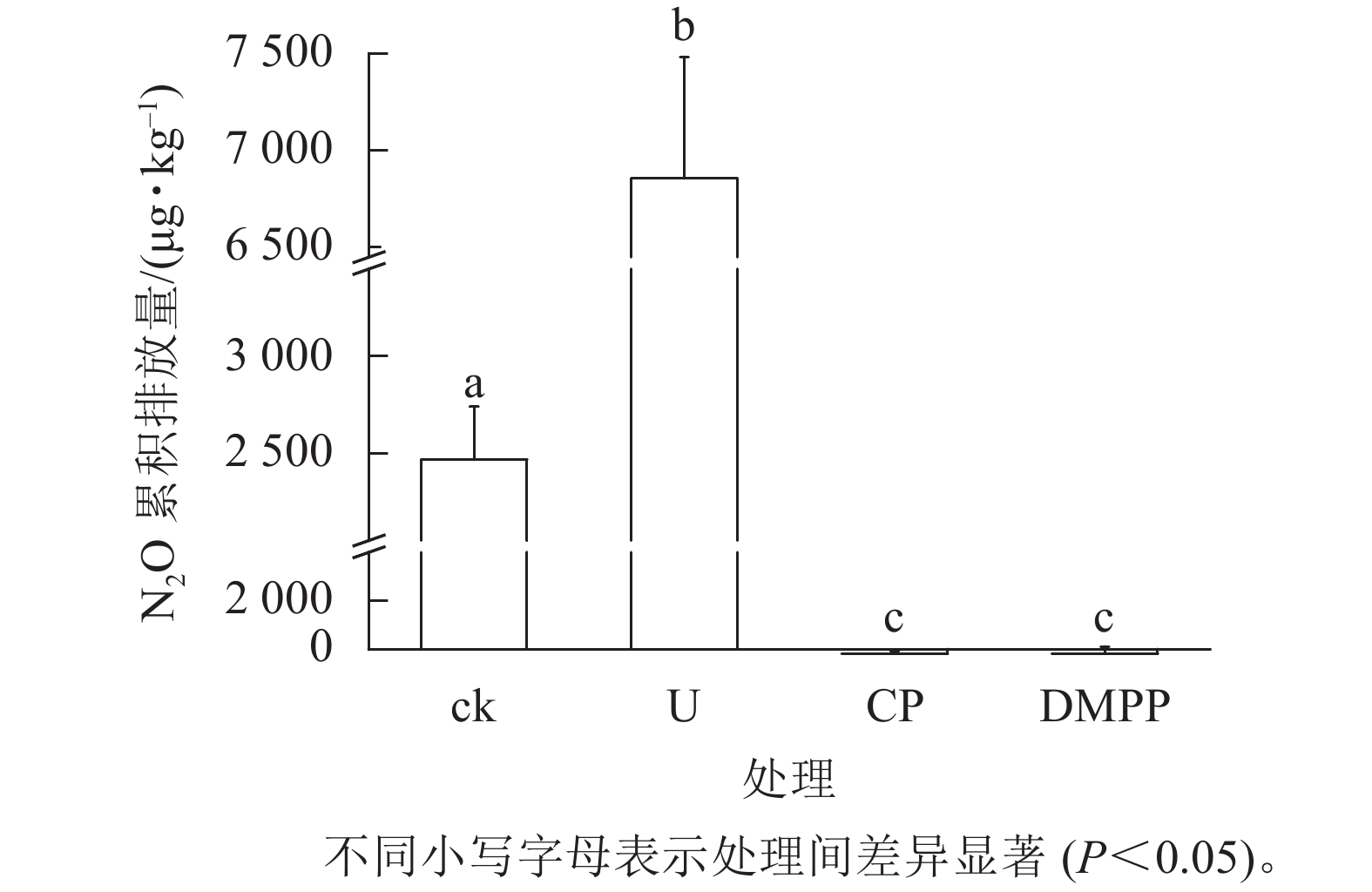
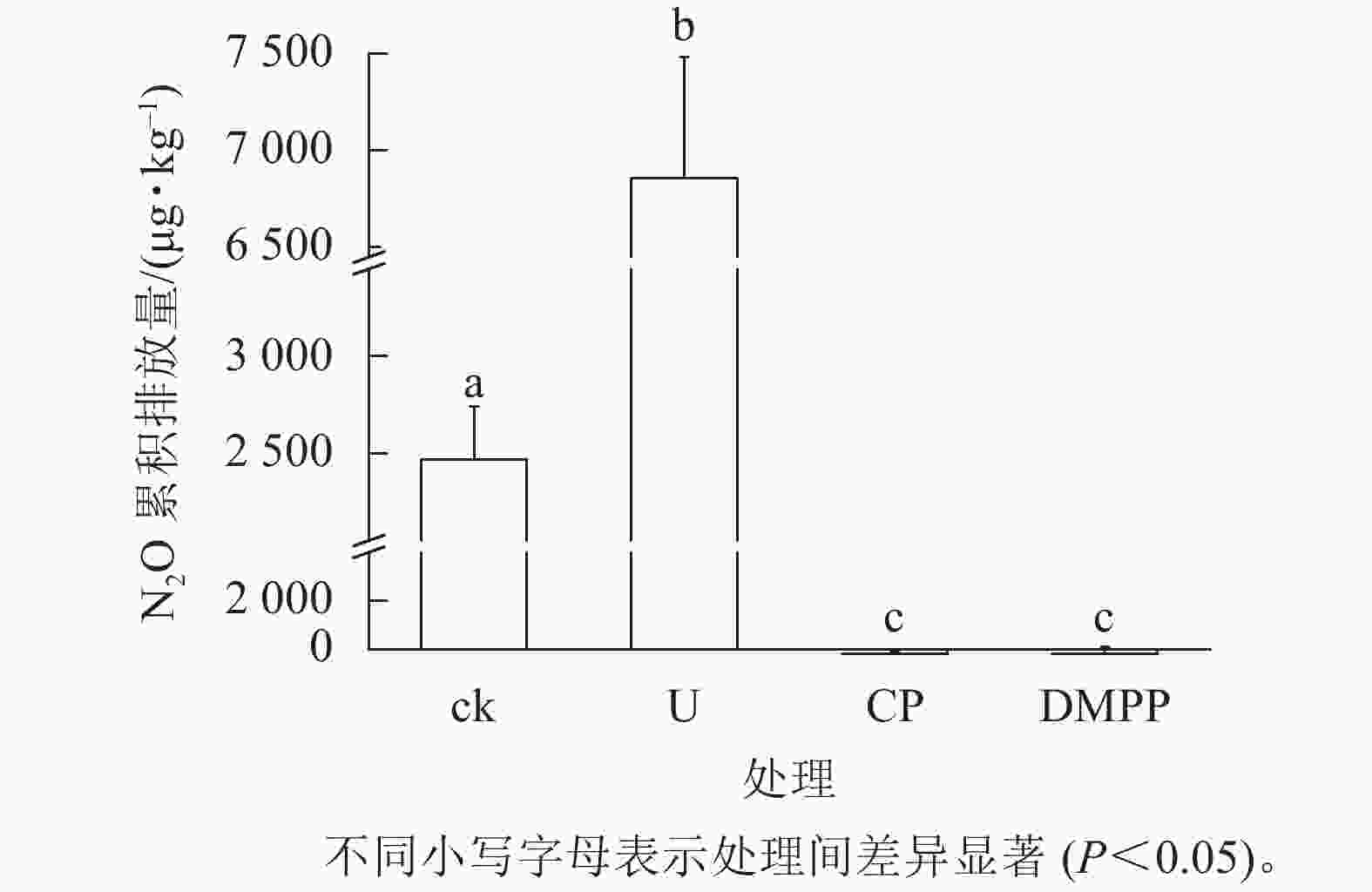
如图3所示:施用尿素显著增加了土壤N2O的累积排放量,是ck处理的3.99倍。但是DMPP与CP处理相较于ck和U处理,显著减少了N2O排放(P<0.05)。
-
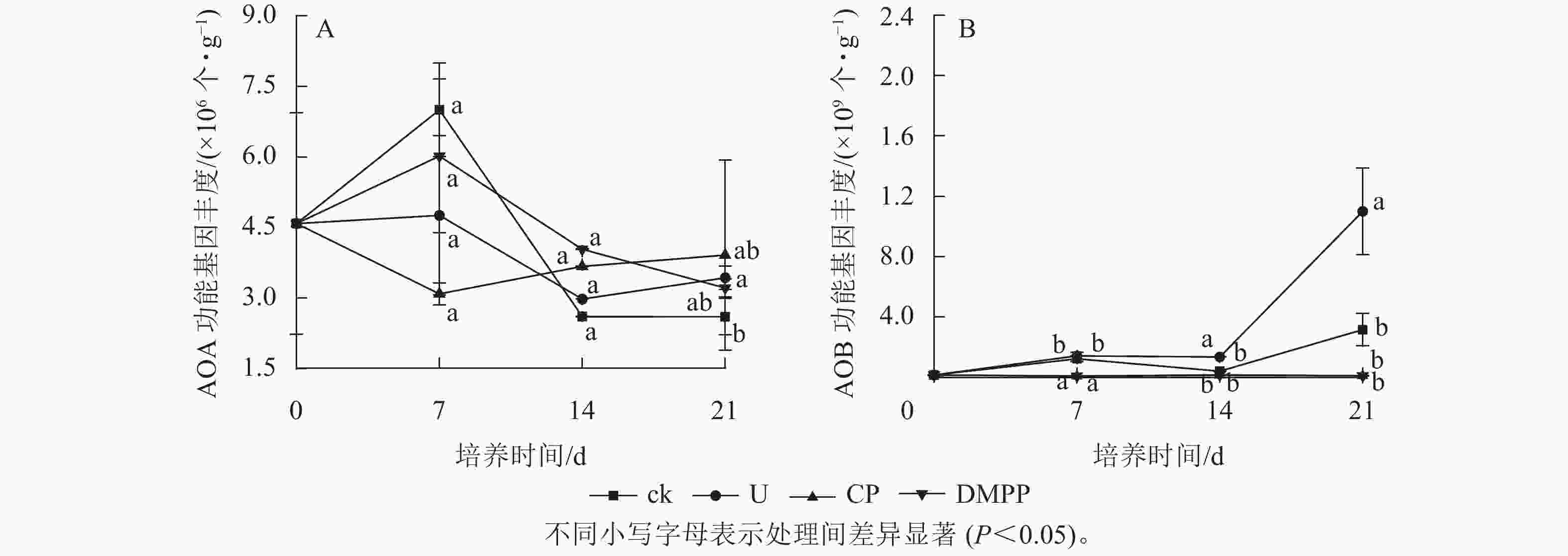
AOA amoA的基因拷贝数为2.6×106~7.1×106 个·g−1,而AOB amoA的基因拷贝数为6.2×106~1.5×109个·g−1(图4),表明土壤中AOA的丰度远低于AOB。与ck处理相比,尿素的添加提高了AOB的丰度,而DMPP和CP的施用显著降低了AOB的丰度(P<0.05)。尿素和硝化抑制剂的使用对AOA的基因丰度无显著影响。培养前14 d,尿素与ck处理的AOB基因丰度都经过了先增后降的过程,在培养的后半段(14 d以后),U处理的AOB数量开始快速增加,到培养结束时达5.5×106个·g−1,较ck处理(1.6×106 个·g−1)提高了249.5%。与U处理相比,2种硝化抑制剂处理的AOB丰度都保持在极低的水平,且2种硝化抑制剂之间的作用效果没有显著差异。
-
如表3所示:N2O排放量与土壤NH4 + -N质量分数,AOB丰度呈正相关,且都达极显著水平(P<0.01);与土壤pH呈负相关,达显著水平(P<0.05)。土壤pH与土壤NH4 + -N质量分数呈极显著正相关( P <0.05);与土壤NO3 − -N质量分数呈极显著负相关( P <0.01)。
因子 M NH4 +-N NO3 −-N AOA AOB pH M 1 NH4+-N −0.526 1 NO3−-N 0.827** −0.906** 1 AOA −0.046 0.333 −0.248 1 AOB 0.960** −0.452 0.743** 0.012 1 pH −0.600* 0.995** −0.941** 0.313 −0.526 1 说明:M指N2O累积排放量;NH4+-N和NO3−-N分别指两者的质量分数;AOA. 氨氧化古菌丰度;AOB. 氨氧化细菌丰度;*、**分别表示在0.05 、0.01水平上显著相关。 Table 3. Correlation between N2O emissions and soil environmental factors
-
在本研究中,各处理的AOB amoA基因拷贝数为6.2×106~1.5×109个·g−1,远高于AOA amoA基因拷贝数(2.6×106~7.1×106 个·g−1),说明AOB在中性稻田土中是重要的氨氧化微生物,这与前人的研究结果相似[19],即,pH 7.0~8.5内适宜AOB生长,AOB是非酸性土壤中主要的氨氧化微生物。本研究结果还显示:添加尿素显著增加了AOB丰度,而对AOA丰度无显著影响。到培养结束时,与ck处理相比,单施尿素处理中土壤AOB丰度增长了678.56%,而AOA丰度有略微下降;且U处理中AOB丰度是AOA丰度的320.6倍。这与前人的研究结果一致,即AOB更偏向于中性至碱性的土壤[20],且在施用尿素或铵肥的土壤中丰度更高[21]。
土壤硝化作用主要受到氨氧化微生物丰度和活性的制约[19]。本研究发现:硝化抑制剂的添加可以显著提高土壤中NH4 + -N质量分数以及减降NO3 − -N质量分数,将土壤净硝化速率保持在较低水平,表明硝化抑制剂可以有效抑制土壤硝化作用。此外,本研究表明:土壤NO3 − -N质量分数与AOB丰度呈极显著正相关,而与AOA丰度无明显相关;说明AOB在土壤硝化作用方面具有更重要的作用,硝化抑制剂是通过抑制AOB丰度来达到抑制硝化作用的目的,这与BENCKISER等[22]的研究结果一致。刘天琳等[19]的室内培养实验表明:在中性紫色稻田土中,AOB对土壤硝化作用的贡献均值为55.6%,AOA对土壤硝化作用的贡献均值为7.5%,AOB在硝化作用中起主导作用,这与本研究结果相符。朱云飞等[23]研究表明:在pH为7.02的中性稻田土中添加硝化抑制剂,可以有效抑制土壤硝化作用。然而,硝化抑制剂在酸性土壤中的应用效果并不显著。杨剑波等[24]的室内培养显示:DMPP可以降低中性稻田土70.18%的土壤表观硝化率,而在酸性红壤中只降低了14.55%。SHI等[25]也发现硝化抑制剂对酸性草地土壤的硝化作用没有显著影响。硝化抑制剂在酸性土壤中的作用效果不佳可能与酸性土壤硝化强度较弱、土壤质地和有机质含量不同等因素有关[24]。
氨氧化微生物在土壤养分和氧化亚氮排放的生化循环中扮演着至关重要的角色[26−27]。在本研究中,与U处理相比,添加DMPP与CP显著降低了AOB丰度,而对AOA丰度无显著影响。同时,AOB丰度与N2O排放量呈极显著的正相关关系。这些结果进一步证实,硝化抑制剂是通过降低AOB丰度来抑制硝化作用和减少N2O排放。这与FAN等[28]的研究结果一致。AOA和AOB对于硝化抑制剂会有不同反应,这可能与AOA的混合营养生长以及2种微生物不同的酶系统有关[27]。SHEN等[29]研究发现:由于细胞代谢的差异,AOB对硝化抑制剂会更加的敏感。硝化抑制剂在减少N2O排放方面的有效性在不同的施用环境中差异很大,这主要归因于pH、有机质、土壤质地等非生物因素以及氨氧化微生物等生物因素2个方面[30]。FAN等[28]研究表明:土壤理化性质是决定硝化抑制剂在N2O减排方面的主要因素,其中土壤pH又发挥着至关重要的作用。在本研究也得出了一致的结果,本研究培养对象是pH为7.17的中性稻田土,结果表明硝化抑制剂在中性稻田土中可以有效减少土壤N2O的排放。DMPP在碱性石灰性土壤中的研究表明:DMPP可以减少石灰性土壤中56%~77%的N2O排放[31]。孙祥鑫等[32]研究发现:硝化抑制剂可以有效减少中性稻田土中74.9%的N2O累计排放量。然而FRIEDL等[33]研究显示:硝化抑制剂DMPP对澳大利亚牧场中酸性土壤N2O的累积排放量没有明显影响,这可能是由于较低的pH会干扰蛋白质的组合或者直接影响酶的活性,在转录后影响N2O还原酶,减少N2O的排放,从而限制了硝化抑制剂的效果[34]。
-
施用尿素显著增加了中性稻田土中AOB的丰度,并促进了土壤N2O的排放。2种硝化抑制剂(DMPP和CP)的添加可以显著降低中性水稻土的净硝化速率,有效抑制土壤硝化作用,并通过降低土壤AOB丰度来减少N2O的排放。
Effects of nitrification inhibitors on soil N2O emission and nitrification in a paddy soil
doi: 10.11833/j.issn.2095-0756.20220605
- Received Date: 2022-08-27
- Accepted Date: 2023-03-28
- Rev Recd Date: 2023-03-21
- Available Online: 2023-07-13
- Publish Date: 2023-08-20
-
Key words:
- paddy soil /
- 3, 4-dimethylpyrazole phosphate (DMPP) /
- 2-chloro-6 -trichloromethyl pyridine (CP) /
- nitrous oxide /
- ammonia oxidizing microorganism
Abstract:
| Citation: | ZHANG Wenhan, CHEN Zhaoming, ZHANG Jinping, et al. Effects of nitrification inhibitors on soil N2O emission and nitrification in a paddy soil[J]. Journal of Zhejiang A&F University, 2023, 40(4): 820-827. DOI: 10.11833/j.issn.2095-0756.20220605 |










 DownLoad:
DownLoad: