-
单核细胞增生性李斯特氏菌Listeria monocytogenes是一种重要食源性人畜共患致病菌[1],侵入机体后可穿过肠道屏障,经淋巴和血液循环进入肝脏和脾脏,随后到达大脑和胎盘[2],引起败血症、脑膜炎以及早产或流产等症状[3]。单核细胞增生性李斯特氏菌广泛存在于牛奶、蔬菜和动物性食品等供应链中,由其引发的食物中毒病例逐年增多,虽然人类感染单核细胞增生李斯特氏菌发病率相对较低,但一旦感染死亡率可达30%~70%,占食源性病原菌感染死亡的半数以上[4]。单核细胞增生性李斯特氏菌分泌的胞外蛋白在细菌感染宿主中发挥着重要作用。细菌存在多种分泌系统将效应蛋白转运至胞外,其中双精氨酸转运系统(twinagininetranslocation,Tat)是运输完全折叠蛋白质的一种分泌系统[5]。
双精氨酸转运系统D (twinagininetranslocation D,TatD)是一种高度保守蛋白,广泛存在于微生物中,可作为一种重要的毒力因子参与修复DNA、降解胞外诱捕网和诱发细胞程序性凋亡[6]。伊氏锥虫Trypanosoma evansi和路氏锥虫Trypanosoma lewisi可分泌TatD蛋白,降解巨噬细胞胞外诱捕网以对抗宿主的固有免疫反应[7]。毛福超[8]研究发现:单核细胞增生性李斯特氏菌TatD重组蛋白具有核酸酶活性,同时通过重组自杀性质粒介导的等位基因交换技术获得了tatD基因缺失菌株(LM10403sΔtatD),但关于单核细胞增生性李斯特氏菌tatD基因对动物的毒力和肠道菌群影响仍不清楚。因此,本研究以小鼠Mus musculus为试验动物,将LM10403sΔtatD口服感染小鼠,观察tatD基因缺失后的单核细胞增生性李斯特氏菌对小鼠毒力和肠道菌群多样性的影响,为进一步探究tatD基因在单核细胞增生性李斯特氏菌与宿主互作中的具体作用以及减毒疫苗研究提供科学依据。
-
单核细胞增生性李斯特氏菌亲本菌株(LM10403s)、缺失菌株(LM10403sΔtatD)和互补菌株(LM10403sCΔtatD)存于洛阳市活载体生物材料与动物疫病防控重点实验室,6周龄雌鼠购自郑州中原动物实验中心。琼脂购自生工生物工程(上海)股份有限公司。卡那霉素购自金克隆(北京)生物技术有限公司。脑心膜浸出液(BHI)肉汤购自青岛海博生物技术有限公司。粪便基因组DNA提取试剂盒购自天根生化科技(北京)有限公司;TksGflex DNA Polymerase购自宝日医生物技术(北京)有限公司;DNA凝胶快速纯化试剂盒购自北京全式金生物技术有限公司。
-
将单核细胞增生性李斯特氏菌LM10403s和LM10403sΔtatD接种于BHI固体培养基上复苏,将LM10403sCΔtatD接种于含卡那霉素的BHI固体培养基上复苏,37 ℃恒温培养箱中培养12~16 h。挑取LM10403s和LM10403sΔtatD单个菌落接种于BHI肉汤中,挑取LM10403sCΔtatD单个菌落接种于含卡那霉素的BHI肉汤中,37 ℃过夜振荡培养备用。
-
用无菌生理盐水将新鲜培养的LM10403s、LM10403sΔtatD和LM10403sCΔtatD洗涤后进行连续10倍稀释,选取5个稀释度的细菌分别给予小鼠口服灌胃,每个稀释度10只小鼠,每只100 μL,灌胃前12 h禁食禁水,接种2 h后正常饲喂。以给予生理盐水的小鼠作为对照,持续观察各处理组小鼠症状直至无小鼠死亡。将死亡小鼠的肝脏无菌接种到BHI固体培养基上,用单核细胞增生性李斯特氏菌引物进行PCR扩增鉴定。采用Bliss法得到菌株对小鼠的半数致死量(LD50)。
-
用无菌生理盐水将新鲜培养的LM10403sΔtatD洗涤后调整细菌浓度,以1.00×105 CFU的剂量给予小鼠口服灌胃,灌胃前12 h禁食禁水,接种2 h后正常饲喂。于首次免疫7 d后按相同剂量进行第2次免疫,第2次免疫7 d后以给予1.00×1010 CFU的剂量对小鼠进行亲本菌株的攻毒。随后持续观察直至连续7 d无小鼠死亡,记录各处理组小鼠症状及死亡情况。以未免疫强毒攻毒组和健康组为对照评价tatD基因缺失菌株的免疫保护效果。
-
将40只6周龄昆明雌鼠适应性饲养7 d,随机平分为磷酸缓冲盐溶液(PBS)、LM10403s、LM10403sΔtatD和LM10403sCΔtatD处理组。分别灌胃200 μL含1.00×106 CFU的菌液,对照组小鼠灌胃200 μL无菌PBS,灌胃前12 h断食,并口服体积分数为10%的Na2HCO3中和胃酸。灌胃24 h后采用颈椎脱臼剖杀小鼠,立即收集肠道内容物。
-
利用粪便基因组试剂盒提取基因组DNA,采用质量浓度为1%的琼脂糖凝胶电泳和Nanodrop 2000测定DNA浓度。以基因组DNA为模板,针对细菌V3~V4作为目标区域进行扩增。引物:343F (5′-TACGGRAGGCAGCAG-3′)和798R (5′-AGGGT ATCTAATCCT-3′)。琼脂糖凝胶电泳检测PCR扩增产物,磁珠法纯化PCR产物。送往上海欧易生物医学科技有限公司利用Illumina Hiseq 2500 (PE250)系统进行高通量测序。
-
高通量测序得到的4组小鼠原始图像数据文件经碱基识别,分析转化为原始双端序列。利用Trimmomatic (v 0.35)、FLASH (v 1.2.11)、split_libraries (v 1.8.0)和UCHIME (v 2.4.2)等软件对原始序列进行质控,得到优质序列。使用Vsearch (v 2.4.2)软件按照97%的相似度进行操作分类单元(OTU)分类[9],并进行Alpha和Beta多样性分析以及菌群构成分析。比较4个处理组小鼠肠道菌群在门和属水平的分布差异。采用PICRUSt软件结合京都基因及基因组百科全书(KEGG)分析各组小鼠肠道菌群信号通路富集情况。
-
采用GraphPad Prism (v 8.0.1)软件对数据进行差异性检验,显著性水平为0.05。
-
从表1可见:小鼠经口服接种不同稀释含量的LM10403s、LM10403sΔtatD和LM10403sCΔtatD后,各组均有小鼠死亡,而无菌生理盐水对照组的小鼠均未发生死亡。PCR扩增鉴定死亡小鼠肝脏分离的细菌为单核细胞增生性李斯特氏菌。通过Bliss法计算得到LM10403sΔtatD口服感染小鼠的LD50为8.11×107 CFU,LM10403s的LD50为1.23×107 CFU,LM10403sCΔtatD的LD50为1.94×107 CFU。表明单核细胞增生李斯特氏菌敲除tatD基因后毒力下降。
处理 接种剂
量/CFU死亡数/
只半数致死
量/CFU4.61×109 8 4.61×108 7 LM10403sΔtatD 4.61×107 5 8.11×107 4.61×106 2 4.61×105 1 2.80×109 9 2.80×108 6 LM10403sCΔtatD 2.80×107 5 1.94×107 2.80×106 3 2.80×105 3 4.45×109 8 LM10403s 4.45×108 6 4.45×107 6 1.23×107 4.45×106 5 4.45×105 3 PBS 0 说明:每个稀释度10只小鼠。 Table 1. Strain virulence determination of bacterial strain
-
LM10403sΔtatD经灌胃第2次免疫7 d后,将LM10403s调整为1.00×1010 CFU对小鼠进行攻毒。结果显示:未免疫的LM10403s攻毒组小鼠于攻毒后全部死亡,无菌生理盐水对照组小鼠未发生死亡。而免疫LM10403sΔtatD的接种组共2只小鼠死亡,死亡时间分别为攻毒感染后的第6天和第9天,其余存活小鼠的精神状态良好,免疫保护率为80%。表明单核细胞增生性李斯特氏菌tatD基因缺失菌株能够对亲本菌株感染小鼠产生较好的免疫效果。
-
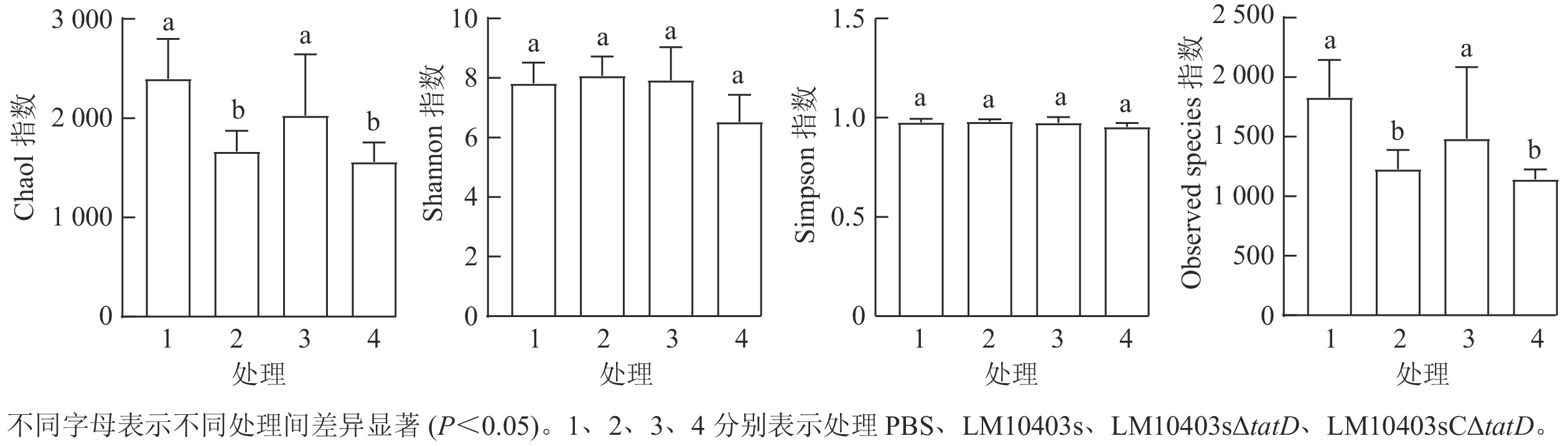
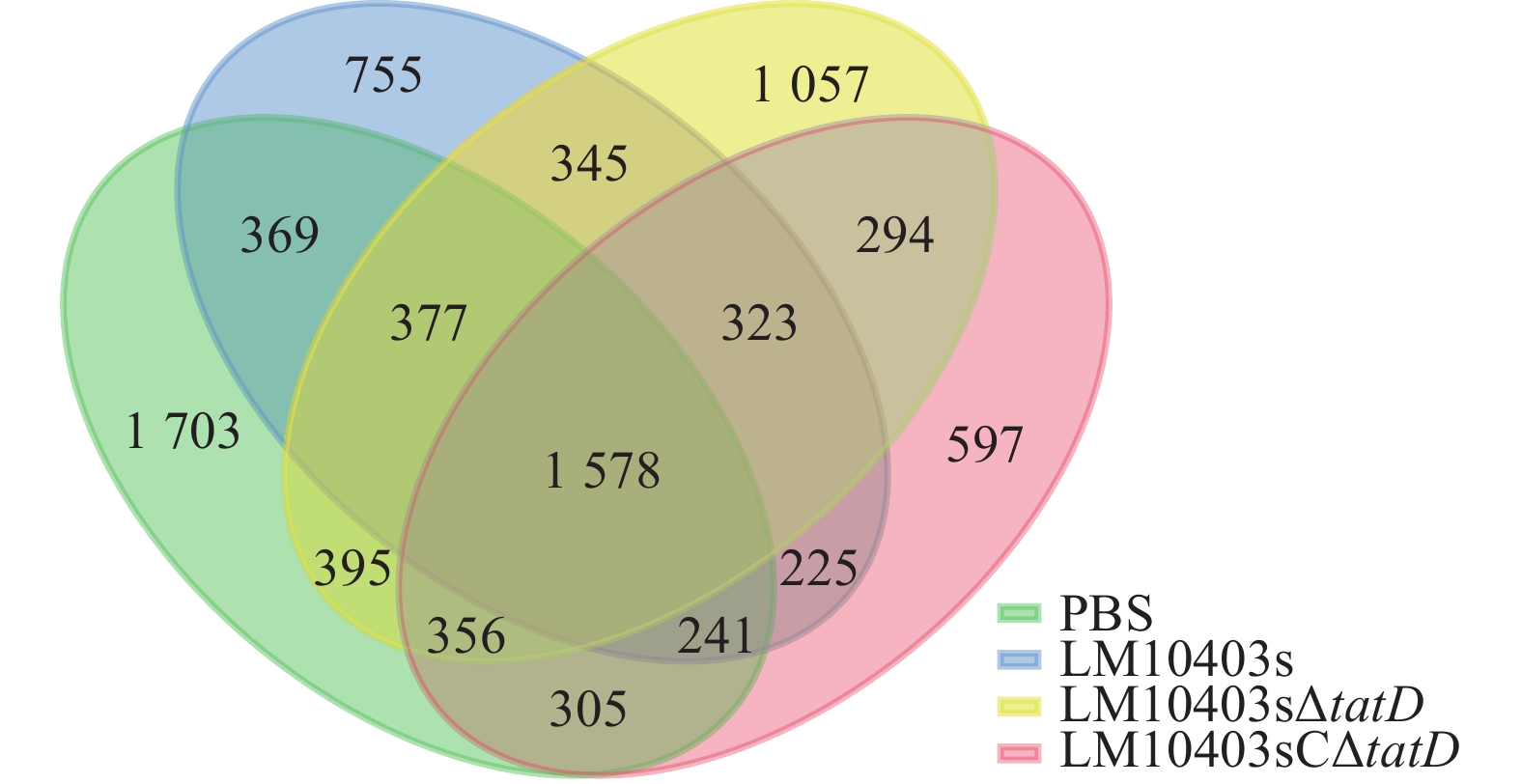
对质控得到的优质序列,按照97%的相似度进行OTU分类,采用维恩图对4个处理组OTU分布情况进行分析。PBS、LM10403s、LM10403sΔtatD和LM10403sCΔtatD 4个处理独有的OTU分别为1 703、755、1 057和597个,4组共有1 781个OTU。肠道菌群多样性由高到低依次为PBS、LM10403sΔtatD、LM10403s、LM10403sCΔtatD处理组(图1)。表明tatD基因缺失后使得单核细胞增生性李斯特氏菌感染小鼠后肠道菌群多样性增加。
-
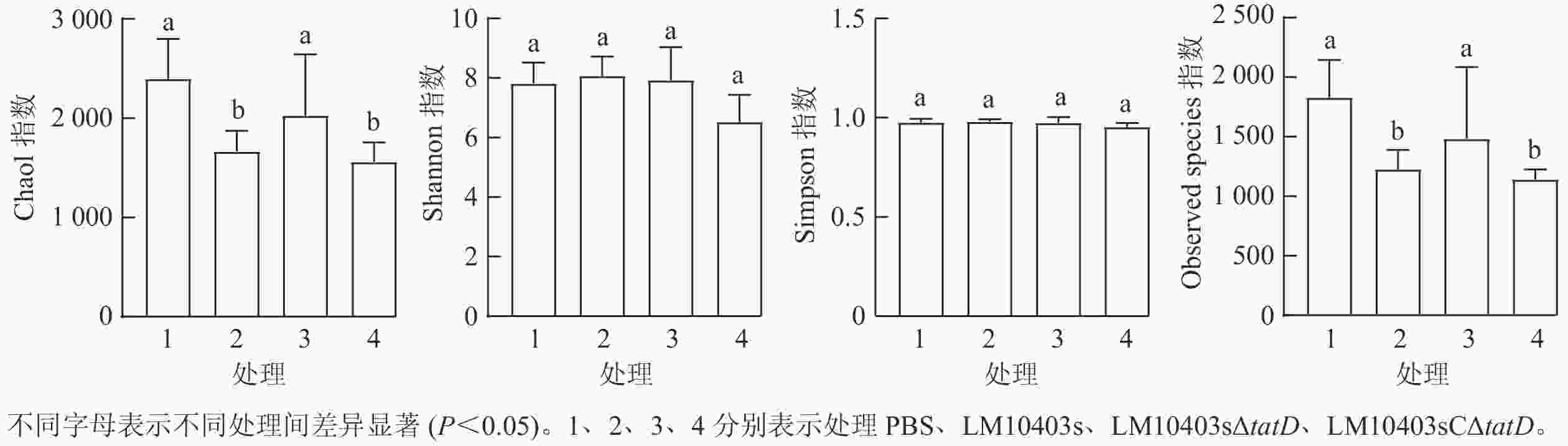
从图2可见:4个处理样品的Shannon指数和Simpson指数差异不显著。Chao1和Observed species指数显示:PBS与LM10403sΔtatD处理组差异不显著,但LM10403s和LM10403sCΔtatD处理组相比于PBS处理组显著下降(P<0.05)。表明各处理小鼠肠道菌群物种均匀度相差不大,但亲本菌株感染小鼠时肠道菌群丰富度显著降低。
-
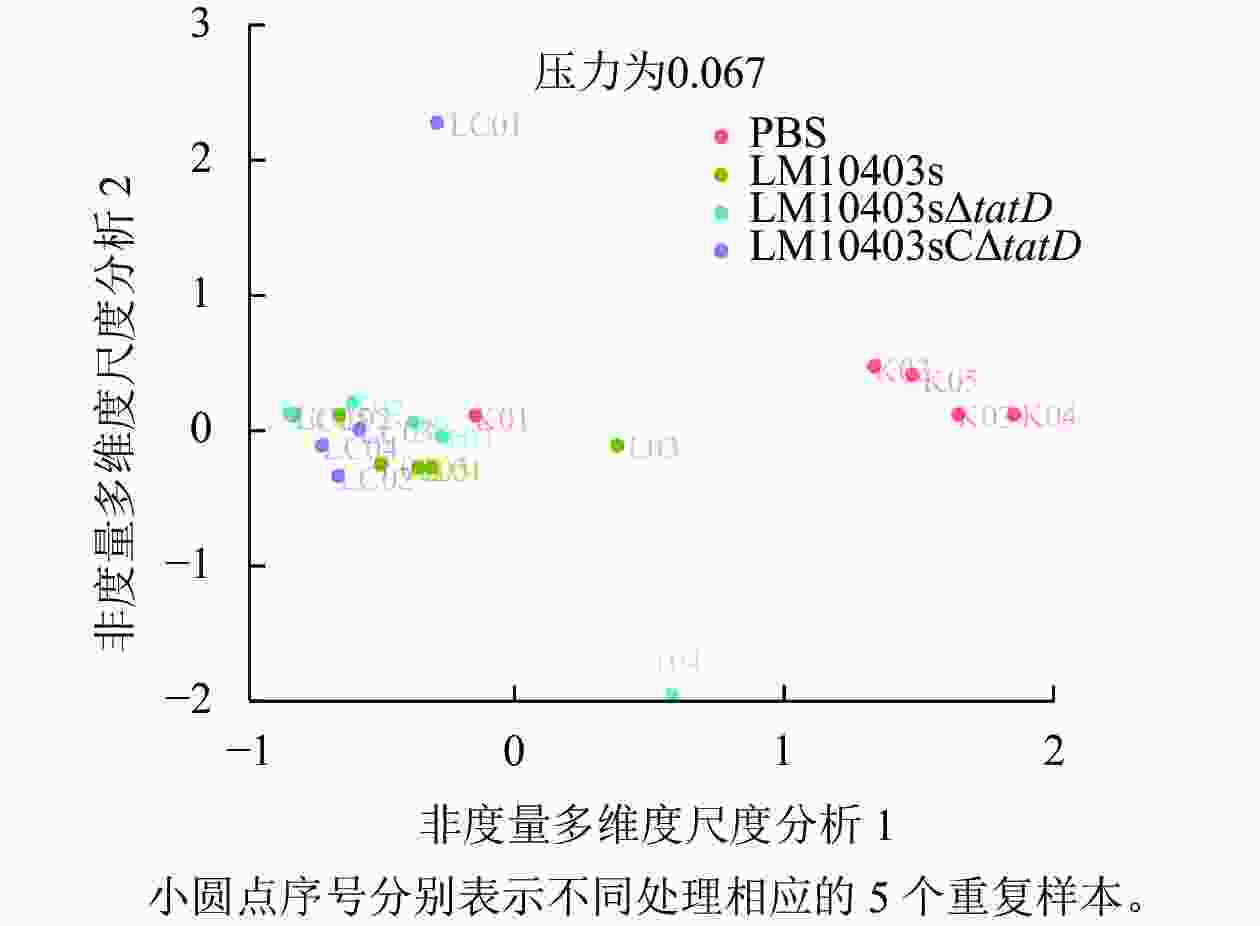
Beta多样性包括多种分析方法,其中非度量多维尺度分析(NMDS)能更好地反映生态学数据的非线性结构,相同颜色为相同分组,同一组的样本距离越近,并与其他组有明显距离,说明分组效果好。图3显示:PBS与各处理组样本距离较远,LM10403sΔtatD与LM10403s、LM10403sCΔtatD处理组距离相对远,LM10403s和LM10403sCΔtatD处理组样本的聚集相近。表明LM10403sΔtatD与LM10403s、LM10403sCΔtatD处理组群落存在差异,但差异不大。
-
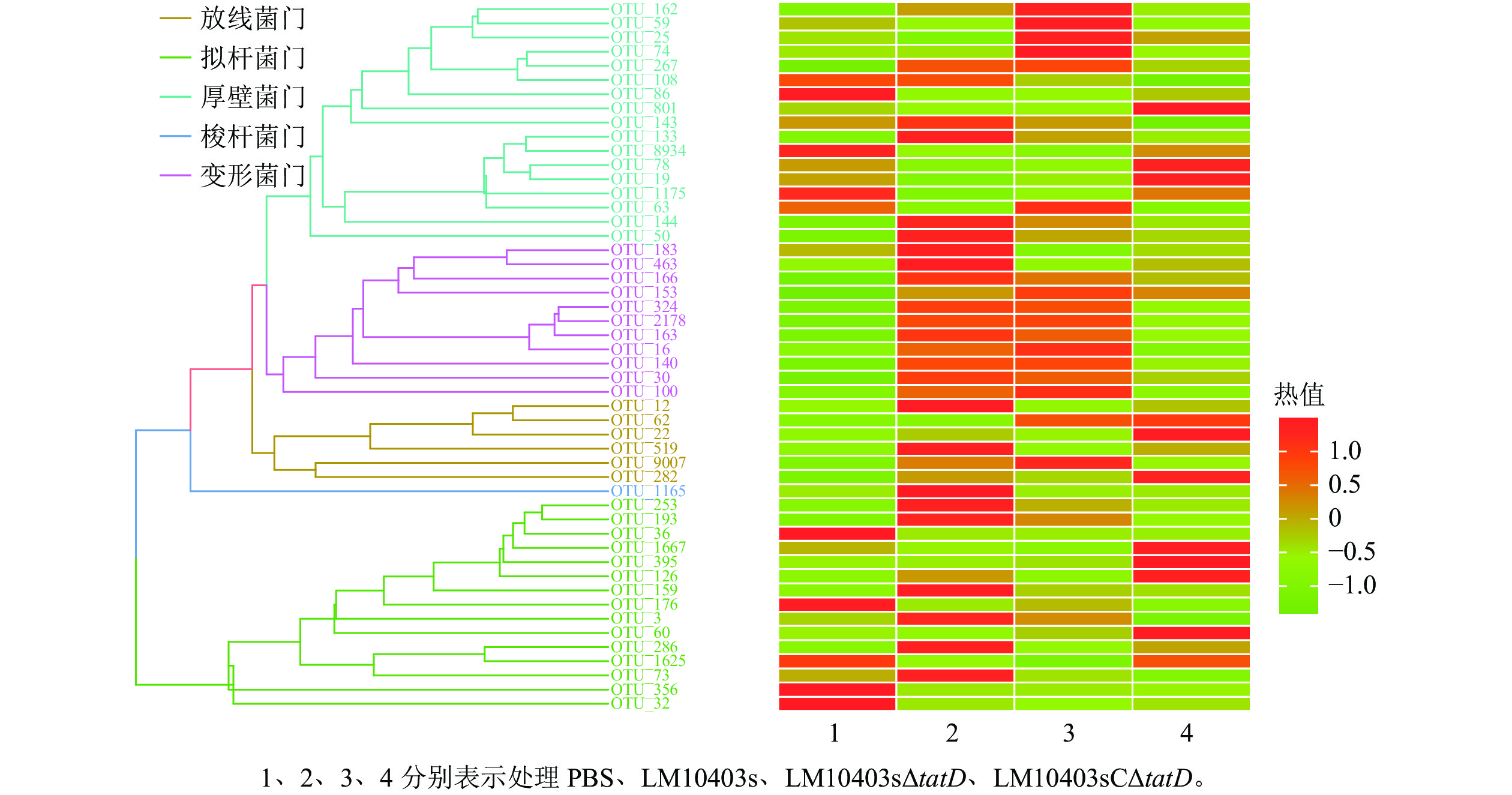
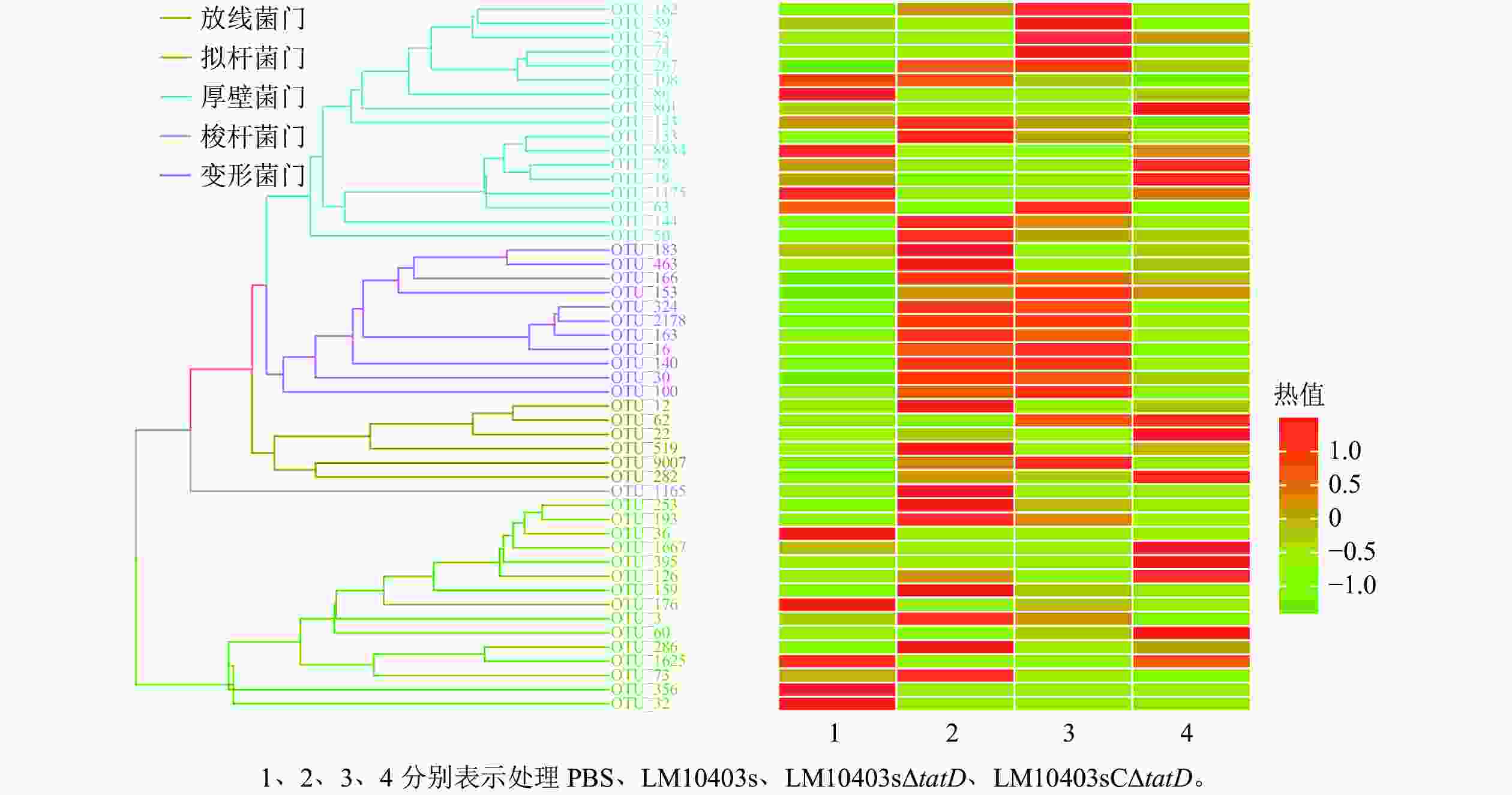
在门水平上,使用平均丰度前50位的门丰度数据绘制热图。结果显示:优势菌门为厚壁菌门Firmicutes、变形菌门Proteobacteria、放线菌门Actinobacteria、梭杆菌门Fusobacteria和拟杆菌门Bacteroidetes。LM10403sΔtatD处理组的厚壁菌门丰度明显高于LM10403s和LM10403sCΔtatD处理组,拟杆菌门明显低于LM10403s和LM10403sCΔtatD处理组(图4)。表明单核细胞增生性李斯特氏菌tatD基因缺失后,厚壁菌门明显增加,且与PBS处理组结果相似。
-
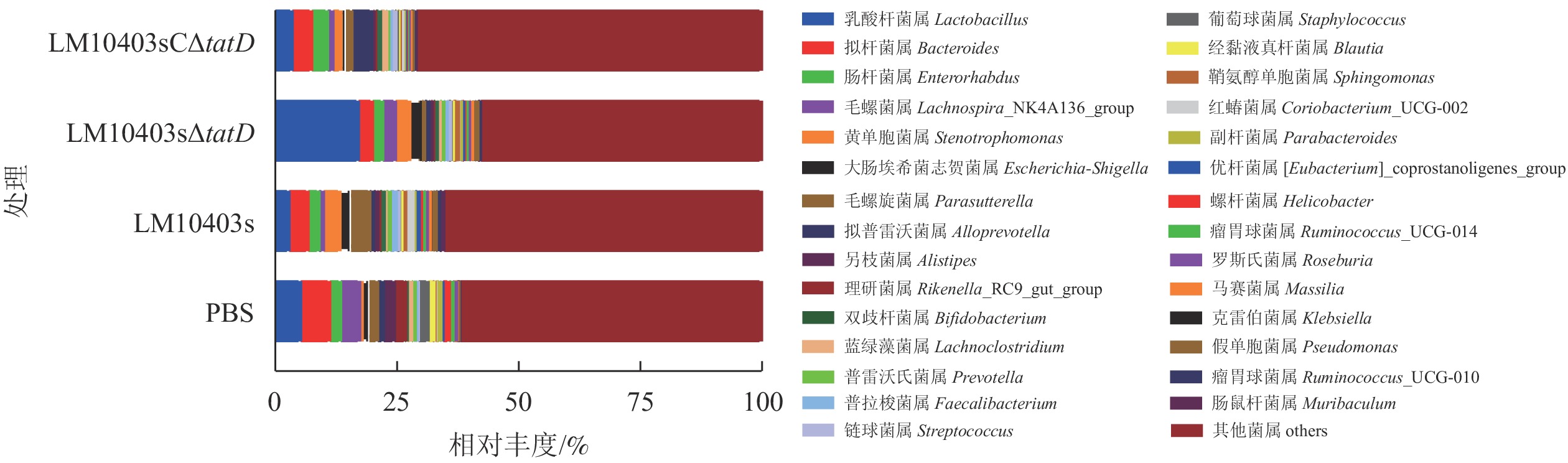
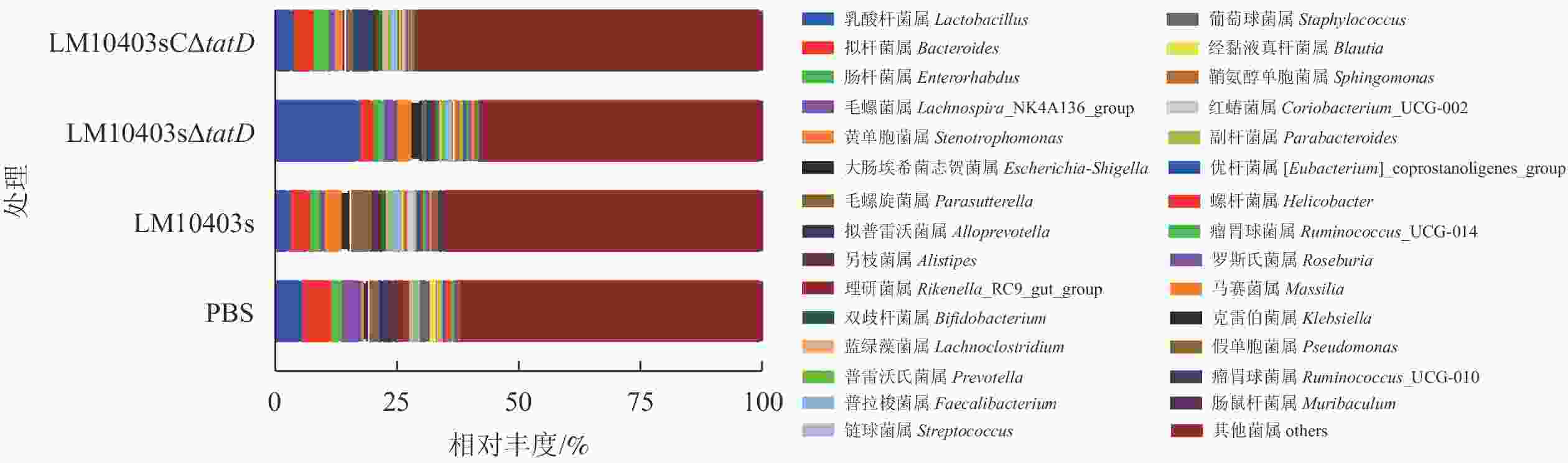
在属水平上,LM10403s、LM10403sΔtatD和LM10403sCΔtatD处理组要由乳杆菌属Lactobacillus、拟杆菌属Bacteroides和肠杆菌属Enterorhabdus组成。LM10403sΔtatD处理组的乳杆菌属相对丰度明显高于LM10403s和LM10403sCΔtatD处理组(图5)。表明tatD基因缺失使单核细胞增生性李斯特氏菌感染的小鼠肠道菌群中乳杆菌属增加。
-
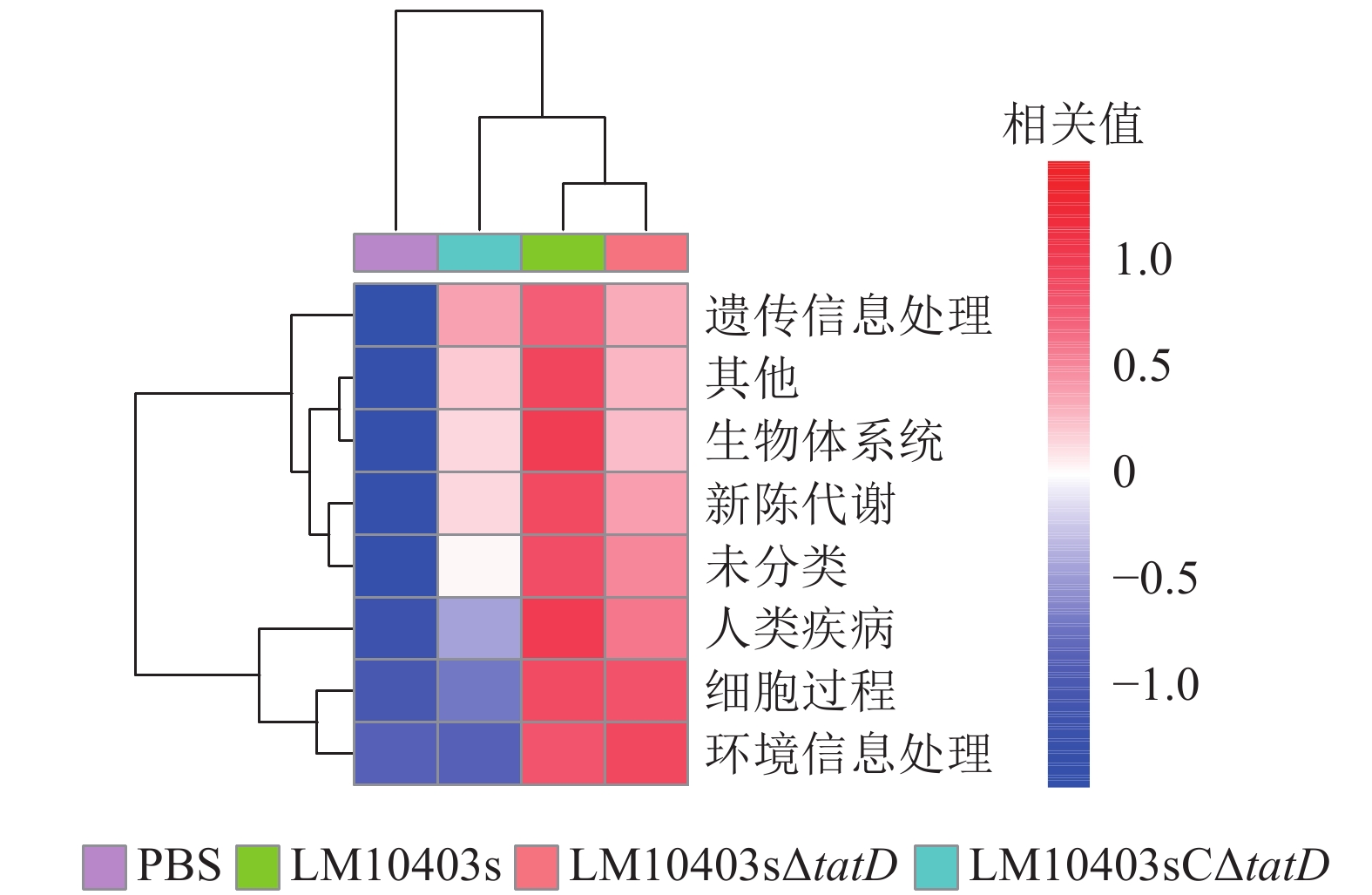
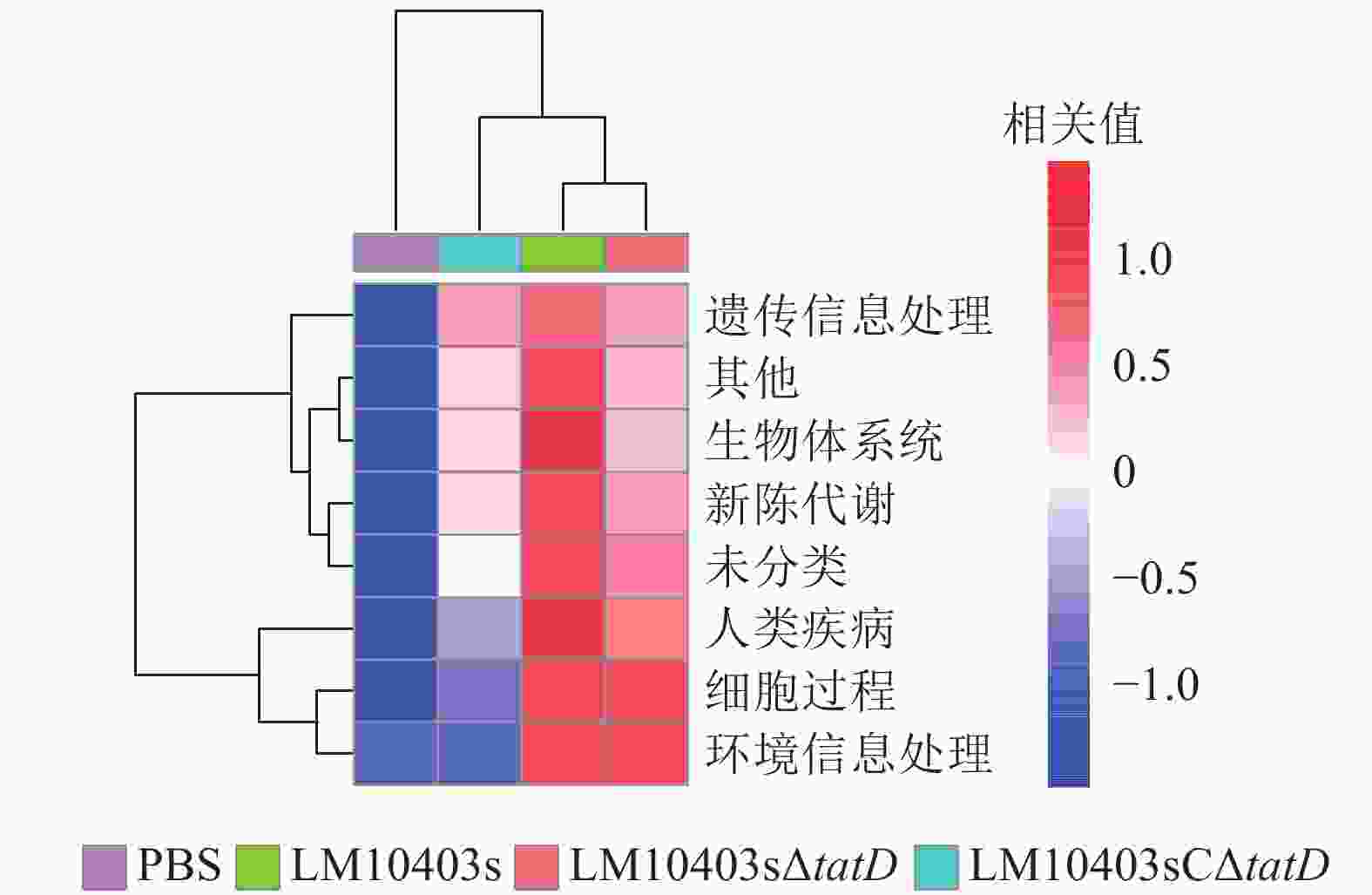
为进一步分析tatD基因缺失后小鼠肠道菌群改变引起的信号通路富集的差异,采用PICRUSt软件结合KEGG,分析4组小鼠在遗传信息处理、生物体系统、新陈代谢、疾病、细胞过程和环境信息处理信号通路富集的差异。结果显示:LM10403sΔtatD和PBS处理组6类生物代谢通路相关性较低,而LM10403s处理组具有较高的相关性(图6)。表明tatD基因缺失可能在单核细胞增生性李斯特氏菌感染小鼠肠道时,影响其细胞过程、新陈代谢等方面。
-
李斯特菌是一种强侵袭性胞内菌,可穿透肠道、血脑和胎盘屏障。自单核细胞增生性李斯特氏菌基因组公布后,大量毒力相关基因已被发掘,并已深入探讨其在单核细胞增生性李斯特氏菌致病性方面的作用[10]。作为重要的效应蛋白转运系统,Tat系统在单核细胞增生性李斯特氏菌穿越宿主肠道屏障过程发挥何种作用仍不清楚。因此,在本研究前期获得单核细胞增生性李斯特氏菌缺失菌株LM10403sΔtatD的基础上进一步探究其口服感染小鼠的毒力,同时采用高通量测序的方法观察其对小鼠肠道菌群的影响。
毒力是微生物对宿主造成损害的相对能力,更是微生物得以在宿主体内传播的主要因素之一[11]。单核细胞增生李斯特氏菌感染过程中,包括Tat分泌系统在内的转运系统调控释放大量效应蛋白[12]。ZHANG等[13]将化脓隐秘杆菌Trueperella pyogenes菌株tatD基因敲除后,缺失菌株在小鼠脾脏的细菌载量以及毒力明显较低。本研究也观察到了单核细胞增生性李斯特氏菌tatD基因缺失后,口服感染小鼠的LD50由1.23×107 CFU降为8.11×107 CFU。JHELUM等[14]也发现:tatD基因缺失的肺炎链球菌Streptococcus pneumoniae在肺、血液和脾脏中负荷的细菌较少,并且对肺脏的病理损伤也较小,证实了tatD基因缺失后肺炎球菌毒性降低。NrfC和NapG蛋白属于铁硫蛋白,铁硫蛋白家族中的其他成员在促进细菌代谢中发挥了重要的作用,并能促进细菌在更严酷的环境中得以生存[15−16],这2个蛋白在野生型菌株中迅速降解,但MATOS等[16]将大肠埃希菌Escherichia coli MC4100菌株的tatD基因敲除后,发现NrfC和NapG蛋白在tatD基因缺失菌株中高度稳定。因此,这也有助于理解tatD基因缺失后,单核细胞增生性李斯特氏菌的毒力下降不大,但这种现象还需进一步研究证实。
尹诗恒等[17]为探究松材线虫Bursaphelenchus xylophilus侵染下,松干、松针与根系部位内生细菌菌群的影响,选用高通量测序方法来进行研究。本研究同样采用高通量测序的方法探究单核细胞增生性李斯特氏菌tatD基因缺失对小鼠肠道菌群的影响。肠道菌群测序显示:各处理组小鼠肠道菌群的物种均匀度相差不大,但亲本菌株感染小鼠时肠道菌群的丰富度显著降低。动物肠道中的细菌门主要有厚壁菌门、拟杆菌门、放线菌门和变形菌门[18]。厚壁菌门是肠道菌群中的一个优势菌门,且其中包含许多有益菌,例如:芽孢杆菌属Bacillus、肠球菌属Enterococcus、乳杆菌属和乳球菌属Lactococcus等[19]。在门水平上,LM10403sΔtatD处理组的厚壁菌门丰度明显高于LM10403s和LM10403sCΔtatD处理组。乳酸菌是公认的安全食品级微生物,在食物发酵和益生菌的应用中发挥着重要作用[20]。服用乳酸菌制剂的人群与其他人群相比,具有肠道屏障功能增强、肠道菌群平衡、炎症消散加快和免疫力增强等特征[21],表明乳酸菌具有治疗肠道功能紊乱、维持肠道菌群平衡和抵御肠道致病菌等多种作用。在门水平上,LM10403sΔtatD组与PBS处理组的厚壁菌门差异不大。但厚壁菌门包含许多菌属,进一步属水平分析单核细胞增生性李斯特氏菌tatD基因缺失感染对小鼠肠道菌群的影响。LM10403sΔtatD处理乳杆菌属相对丰度为20%,显著高于LM10403s和LM10403sCΔtatD处理组。因此,tatD基因缺失使单核细胞增生性李斯特氏菌感染的小鼠肠道菌群中有益菌增加。KEGG功能预测发现:LM10403sΔtatD和PBS处理组与6类生物代谢通路相关性较低,而LM10403s处理组与6类生物代谢通路相关性较高。因此,tatD基因可能直接或间接参与细胞过程和环境信息处理等信号通路。
综上所述,本研究发现单核细胞增生性李斯特氏菌tatD缺失菌株感染小鼠毒力较亲本菌株明显下降,并且有较好的免疫保护效力。单核细胞增生性李斯特氏菌tatD基因缺失感染小鼠后,肠道菌群多样性高于亲本菌株,且有益菌增加。同时,发现tatD基因可能直接或间接参与细胞过程和环境信息处理等信号通路。
Effect of tatD gene deletion of Listeria monocytogenes on virulence and gut microflora in mice
doi: 10.11833/j.issn.2095-0756.20220758
- Received Date: 2022-12-10
- Accepted Date: 2023-11-03
- Rev Recd Date: 2023-09-30
- Available Online: 2024-01-19
- Publish Date: 2024-02-20
-
Key words:
- tatD gene /
- Listeria monocytogenes /
- virulence /
- 16S rRNA /
- gut microbiota
Abstract:
| Citation: | NIU Junhui, LI Qi, MAO Fuchao, et al. Effect of tatD gene deletion of Listeria monocytogenes on virulence and gut microflora in mice[J]. Journal of Zhejiang A&F University, 2024, 41(1): 161-168. DOI: 10.11833/j.issn.2095-0756.20220758 |










 DownLoad:
DownLoad: