-
石蒜属Lycoris植物为多年生球根花卉,花型奇特且类型繁多,花色丰富,冬春花败观其叶,夏秋叶枯赏其花,观赏价值高[1]。此外,石蒜属植物含有的生物碱、石蒜碱颇具医药研究开发潜力[2−3]。石蒜新品种‘梦幻少女’Lycoris chinensis × radiata ‘Astro Girl’是中国石蒜L. chinensis和石蒜L. radiata的种间杂交种,其花色以乳黄白色为底色,雾粉渐变于花瓣末端,花朵形态优雅折曲,同样渐变色的花丝抽展而出,呈现梦幻、活力、雅致的观赏感,极具研究和推广价值,但‘梦幻少女’染色体数目(2n=19)为非整倍数,自然条件下种球繁殖效率低,速度慢。
植物组织培养技术成为目前推广新品种和良种的重要手段。该技术具有材料耗费量少、繁殖系数高、不受季节限制、周期短和快速批量成苗的特点,能够良好地保持繁殖母本遗传基因型,从而获得优良稳定材料。至今已建立多种石蒜属植物的组培快繁体系[4],但主要以带基盘的双鳞片或鳞茎为外植体,通过诱导不定芽来建立离体快繁体系,繁殖系数相对较低。通过体胚发生途径,可获得数量更多、发育速度更快、遗传性更稳定的再生植株,在栎属Quercus[5]、松科Pinaceae[6−7]及云杉属Picea[8]等植物建立的体胚发生体系,加速了林木育种改良、苗木工程绿化、有效成分提取及种质资源保护与利用等方面的应用。然而,多数植物体胚的研究仍存在体胚形成再生植株困难和发育不同步等瓶颈[9]。关于石蒜属植物体胚相关的研究,仅王彩霞等[10]通过诱导忽地笑Lycoris aurea鳞茎盘端1/3鳞茎的愈伤组织,进而分化体胚,而关于石蒜属其他种的体胚研究还相对较少。鉴于此,本研究分析了石蒜‘梦幻少女’体胚发生过程中的细胞学与生理特性,探索其体胚繁殖体系及发育机理,为石蒜属植物工厂化育苗提供技术支撑。
-
将石蒜鳞茎球不同部位组织为外植体,诱导愈伤组织。在愈伤组织连续增殖继代中,筛选稳定增殖的淡黄色、半透明的胚性愈伤组织,再将胚性愈伤组织进行体细胞胚的诱导与增殖培养,筛选出D23培养基[基础培养基(MS)+2.00 mg·L−16-苄氨基嘌呤(6-BA) +0.05 mg·L−12,4-二氯苯氧乙酸(2,4-D)+30.00 g·L−1蔗糖+6.20 g·L−1琼脂]为最佳的体胚诱导与增殖培养基。培养条件:光周期14 h·d−1,光照1 500 lx,温度(27±2) ℃,以下研究培养条件相同。
2022年7月选取生长一致的胚性愈伤组织,接种至D23培养基进行培养,间隔5 d (0、5、10、15、20、25、30、35和40 d)挑选长势相对一致的材料,体视镜(OLYMPUS SZX 7)显微观察并拍照,同时,取10 g材料液氮速冻后于−80 ℃超低温冰箱中保存,用于生理生化指标测定,重复3次。
-
石蜡切片参照李丹等[11]的方法改良后进行,取材固定3 d以上,梯度乙醇脱水、浸蜡,之后于65 ℃融化石蜡连续浸蜡8 d,新蜡包埋、切片(厚度9 μm)、制片,40 ℃烤片至干燥,常温保存备用。
脱蜡复水,染色(苏木素中染色4~5 min,盐酸水溶液分化5 s,氨水水溶液返蓝30 s内,自来水浸洗),依次于体积分数为85%、95%的乙醇脱水各5 min,伊红染液染色5 min,乙醇脱水,中性树胶封片,于徕卡(LEICA DM4000)显微镜观察拍照。
-
称取0.1 g样品置2.0 mL离心管中,加入1.0 mL磷酸缓冲液(pH 7.4)和适量研磨珠,于−10 ℃预冷的冷冻研磨机中研磨,4 ℃ 8 000 r·min−1离心 20 min,取上清液,冰上保存备用。
采用双抗体一步夹心法酶联免疫吸附试剂盒(GIVEI,极威生物科技公司,上海)测定淀粉、总蛋白质和酶活性[12]。在预先包被抗体的孔板中,依次加样、温浴、清洗、显色后,于酶标仪(SPECTRA MAX 190)上测定D(450),计算样品的质量浓度或活性。
-
参考林绍艳等[13]方法将1.0 g·L−1腐胺、精胺和亚精胺混合标准品溶液,稀释成10.0 mg·L−1,再逐级稀释2、4、8、16、32、64、128倍,上机测定其峰面积,并以多胺质量分数为自变量,峰面积为因变量,绘制标准曲线。
称取0.1 g各样品冻干粉末于离心管中,加入预冷的体积分数为10%的乙腈2.0 mL,涡旋混匀,9 000 r·min−1离心5 min,0.22 μm滤膜过滤,使用超高效液相色谱-串联质谱(UPLC-MS/MS)测量样品的多胺质量分数。
-
采用Excel 2022整理数据,SPSS 23.0对生理生化指标进行单因素方差分析和多重比较(邓肯检验),使用GraphPad prism 9.0制图。
-
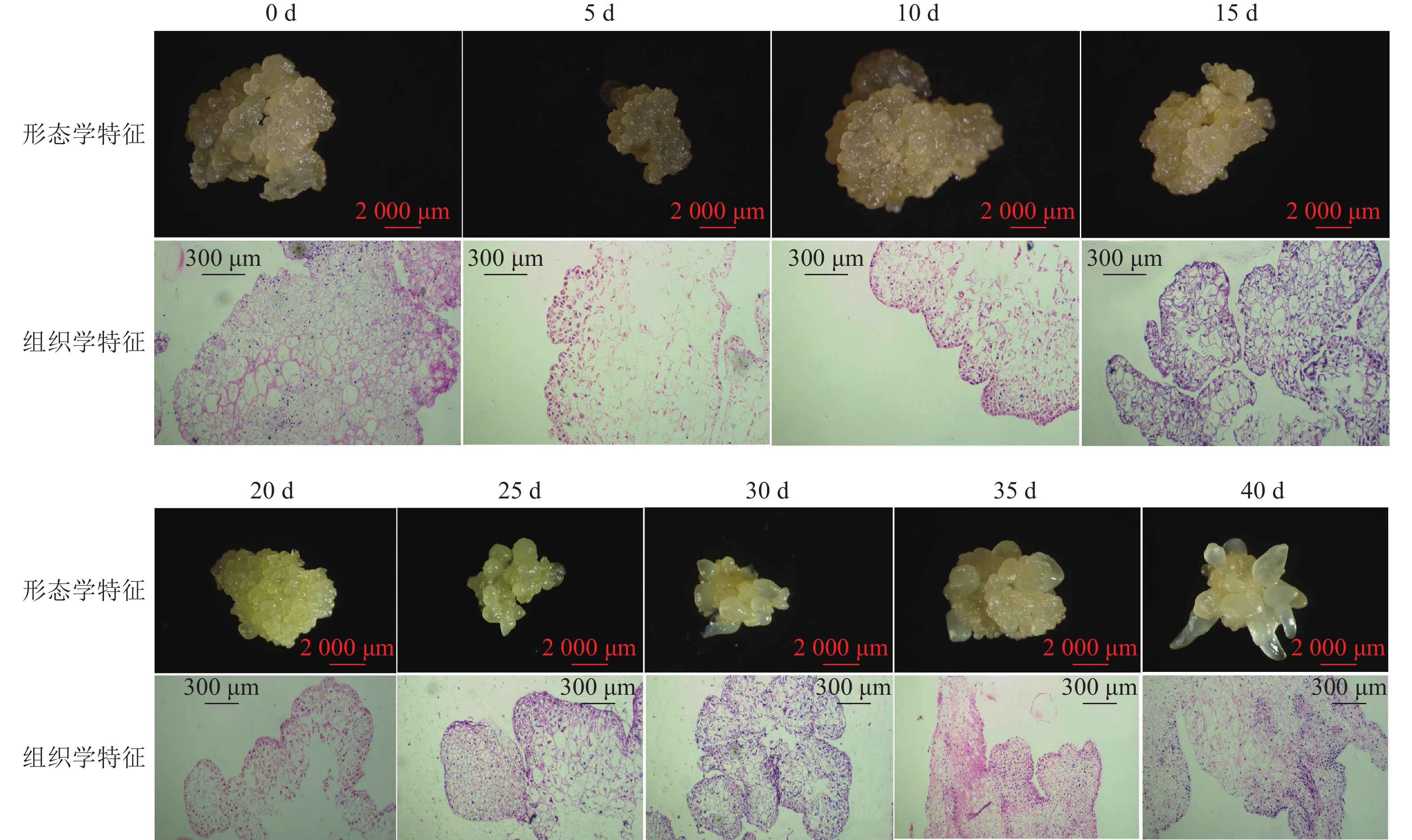
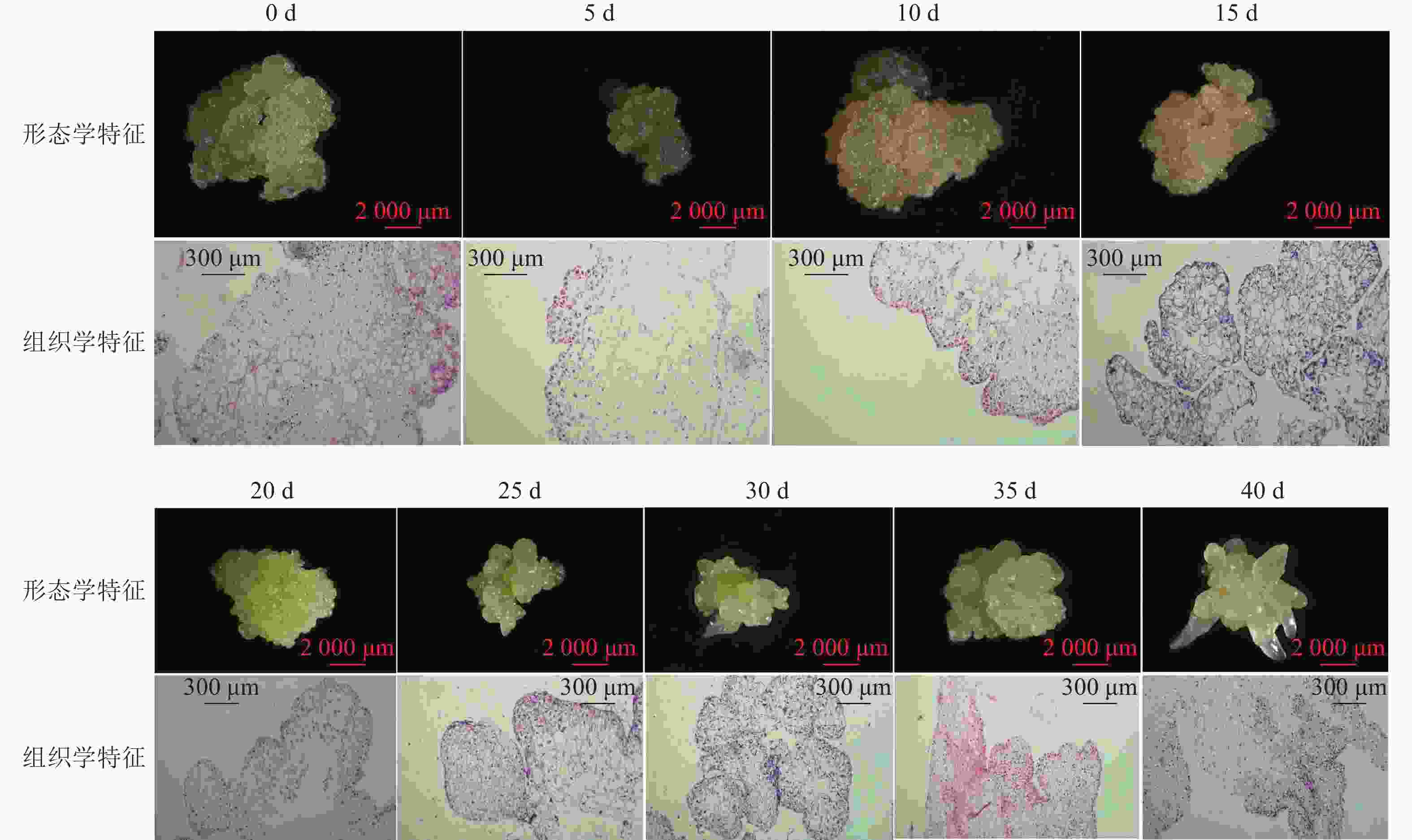
组织学观察表明:在接种的第0~15天时,胚性愈伤组织呈浅黄色透明状。刚接种时,愈伤组织表面为鲜润、淡白色的颗粒状团块,胚性细胞在愈伤组织表层或亚表层散状分布,内层多为较大的非胚性细胞疏松排列;接种第5天时,愈伤组织逐渐适应培养环境,表面湿润,表层附近的胚性细胞开始活跃分裂,且密集分布;第10天时,愈伤组织发生了一定程度的增殖,球形胚初步形成并向外凸出;第15天时,愈伤组织主要发生增殖与分化球形胚,少量心形胚和棒状胚形成,心形胚维管束形成,与母体愈伤组织形成生理隔离(图1)。
在培养的第20~40天时,胚性愈伤组织转变为光亮、浅黄色。在第20天时,愈伤组织继续增殖、分化为球形胚,且球形胚加速膨大,维管束形成;同时,部分球形胚明显分化至心形胚、棒状胚。第25天时,体胚之间的排列开始变得疏松,簇状的体胚形态发育速度不均一。与培养第0~20天相比,体胚形态成熟转化速度明显加快,大部分球形胚发育至心形胚、棒状胚、子叶胚雏形,体积膨大。第30天时,胚性愈伤组织增殖不明显,继续分化少量新的球形胚,大部分体胚发育至子叶胚的初步形态且继续膨大;第35天时,胚性愈伤组织活性开始减弱,簇状体细胞胚的形态发育速度再次加快,差异较明显,同时存在球形胚、心形胚、棒状胚和子叶胚,子叶胚形态轮廓更加成熟,结构上外边缘的胚性细胞分布密集,总体上棒状胚的数量相对较多;第40天时,形体较大的体胚继续成熟,部分体胚退化至非胚性愈伤组织,子叶胚中间分化出具有两极性的轴状胚性细胞团。
在0~40 d的体胚发生过程中,球形胚从发生到短暂膨大维持约20 d,进一步分化为心形胚、棒状胚和子叶胚。在膨大的球形胚后期或心形胚时期基部会形成维管束,后期子叶胚可发育为小鳞茎。
-
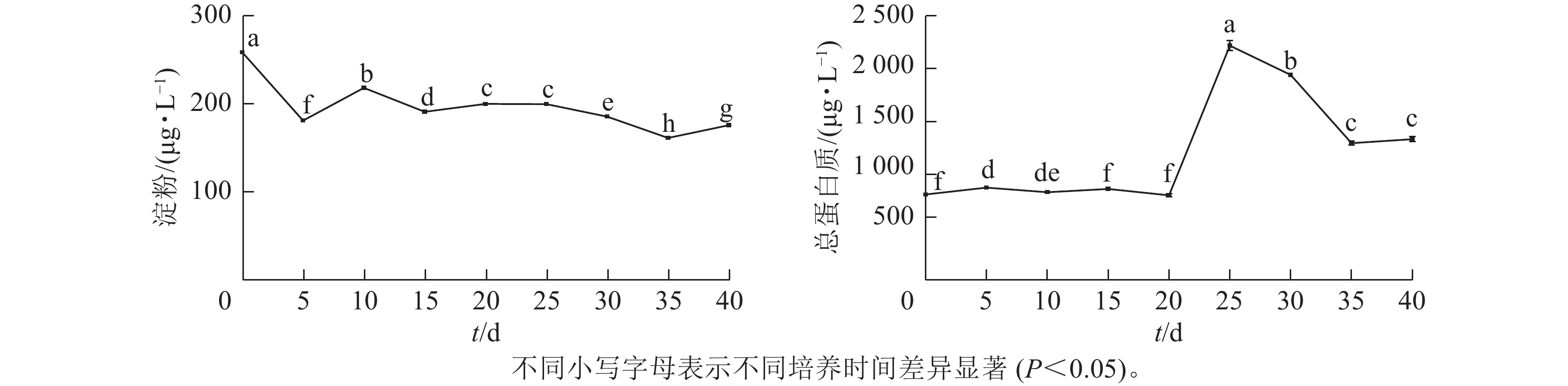
由图2可见:在体胚发生过程中,淀粉质量浓度呈先下降后稳定的趋势。在体胚发生早期(0~20 d)和体胚成熟期(20~40 d),淀粉质量浓度存在显著差异(P<0.05)。
根据体胚发生过程中的形态与组织学特征(图1),在体胚早期(0~20 d),球形胚形成时,淀粉质量浓度发生了明显积累;球形胚发育至心形胚、棒状胚时,淀粉质量浓度大幅下降。在子叶胚时期(20~35 d),随着子叶胚的形成、体积膨大和体胚成熟,淀粉质量浓度持续下降,呈现负相关关系。第35~40天时,可能由于营养供给不足、次生物质积累少等导致部分体胚退化为愈伤组织,部分子叶胚出现明显的两极分化,此时淀粉质量浓度明显上升。
总蛋白质质量浓度在体胚发生早期(0~20 d)时保持不变,在20~25 d迅速上升,而后呈下降趋势。体胚早期发育阶段总蛋白质质量浓度差异不显著,第25天时出现峰值后回落,子叶胚分化到成熟期间(20~40 d)时,总蛋白质质量浓度差异显著(P<0.05)。
在体胚早期(0~20 d),愈伤组织增殖、体胚前期形态转化(球形胚、心形胚和棒状胚)阶段,总蛋白质质量浓度维持在一定的阈值,变化不明显。愈伤组织和体胚混合体为淡黄色(第20天时转为光亮色泽)。子叶胚时期(20~35 d),总蛋白质质量浓度骤升式积累(子叶胚形成期);子叶胚逐渐成熟且膨大时,总蛋白质质量浓度下降,说明子叶胚形成与总蛋白质质量浓度呈正相关,子叶胚成熟阶段则相反;在第35~40天时,总蛋白质质量浓度有所回升。
-
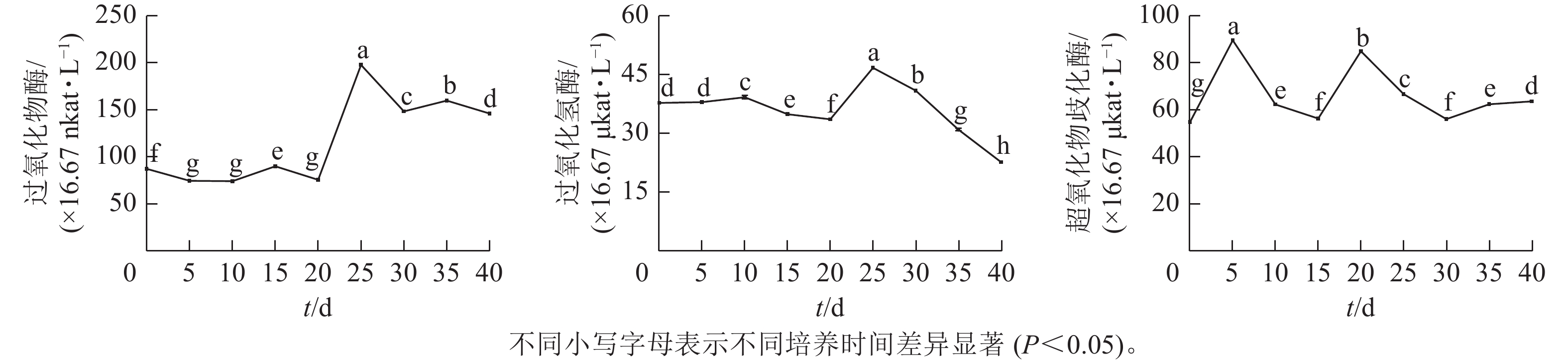
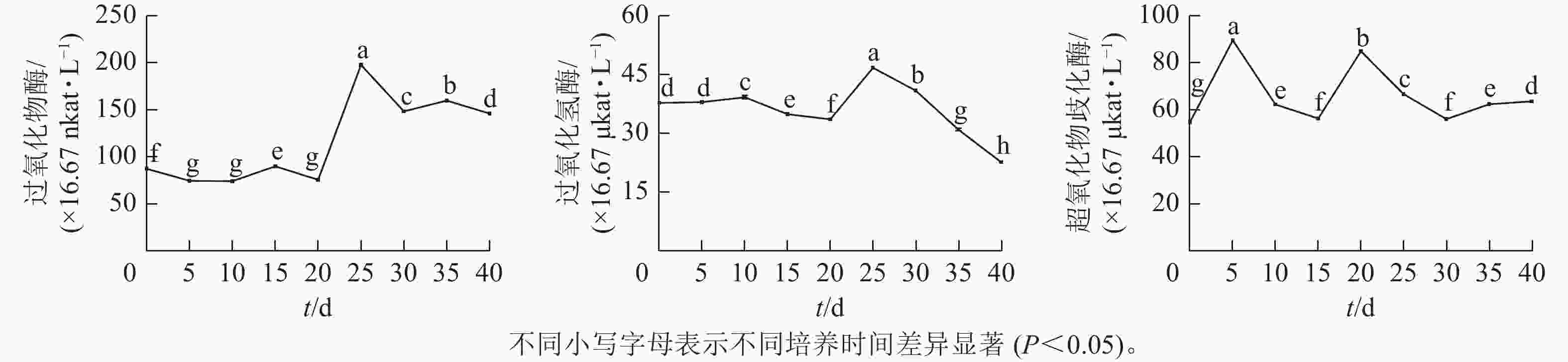
由图3可见:体胚发生过程中,过氧化物酶(POD)、过氧化氢酶(CAT)和超氧化物歧化酶(SOD)活性存在显著差异(P<0.05)。POD和CAT活性变化与总蛋白质质量浓度的变化趋势相似,在体胚发生早期(0~20 d)保持不变,在20~25 d时迅速提高,而后下降。POD活性下降到30 d时保持相对不变;CAT活性在30~40 d时呈持续下降趋势。POD和CAT活性在体胚发生的0~25 d时活性变化与体细胞的形态变化相对应,且和总蛋白质质量浓度变化趋势相似。子叶胚成熟期(25~40 d),POD活性先下降,此时大部分体胚发育至子叶胚且继续膨大(25~30 d),之后子叶胚成熟阶段POD活性变化较小(30~40 d);而CAT活性随着子叶胚的成熟逐渐下降(25~40 d),表明POD和CAT活性与子叶胚的形成呈正相关,POD活性与子叶胚初步膨大呈负相关,CAT活性与子叶胚的成熟呈负相关。
SOD活性呈现2个峰值后略有回升。在培养第5天和第20天时出现明显峰值,体胚分化早期(0~15 d)和分化中后期(15~40 d),SOD活性差异显著(P<0.05)。在体细胞胚早期(0~15 d),胚性愈伤组织处于增殖阶段中,SOD活性提高;在球形胚的形成与增殖阶段中,SOD活性逐渐下降。在心形胚和棒状胚的形成阶段(15~20 d),SOD活性提高。在子叶胚的形成及成熟阶段(20~40 d),SOD活性急剧下降;当内部聚集性的两极开始形成时,SOD活性又有所回升。
-
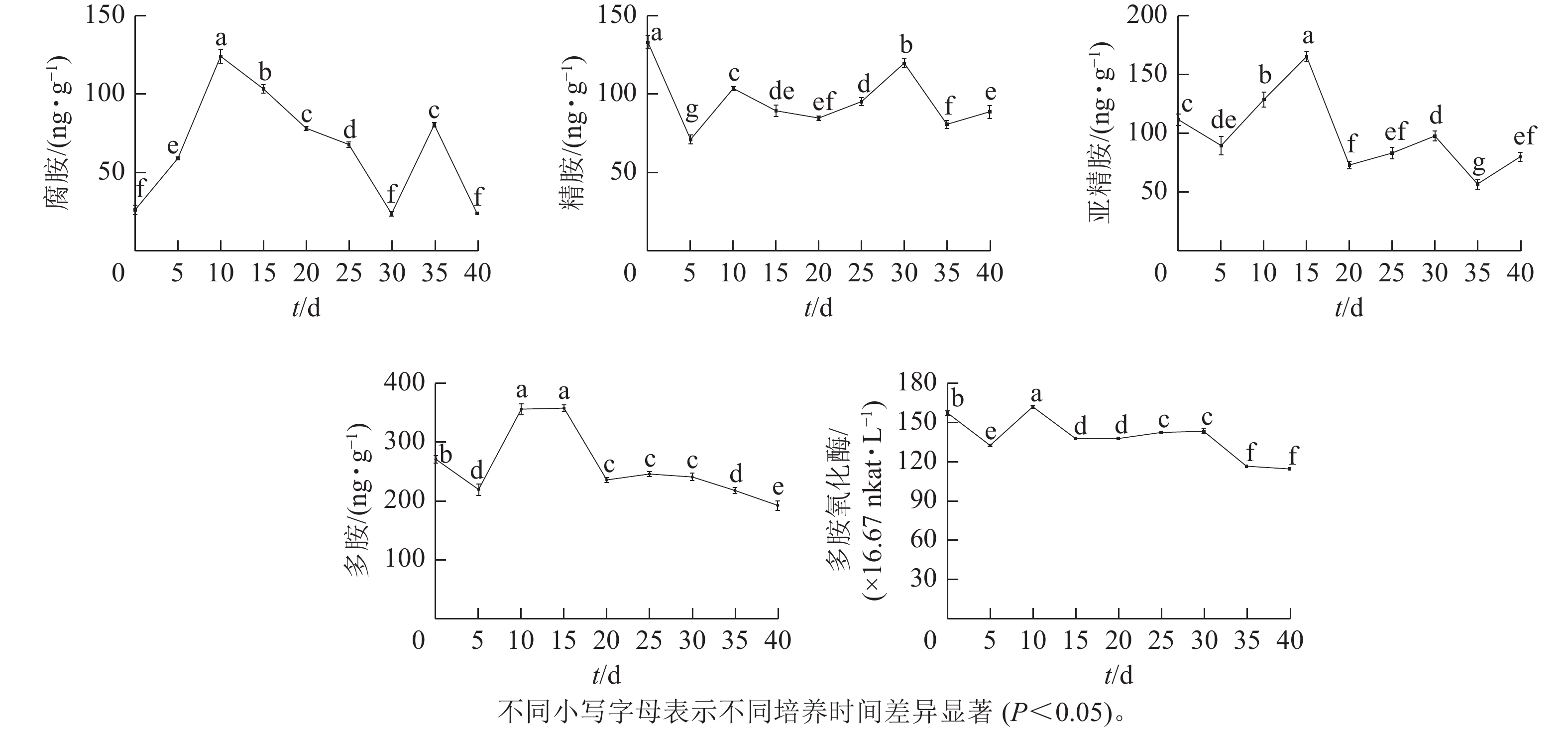
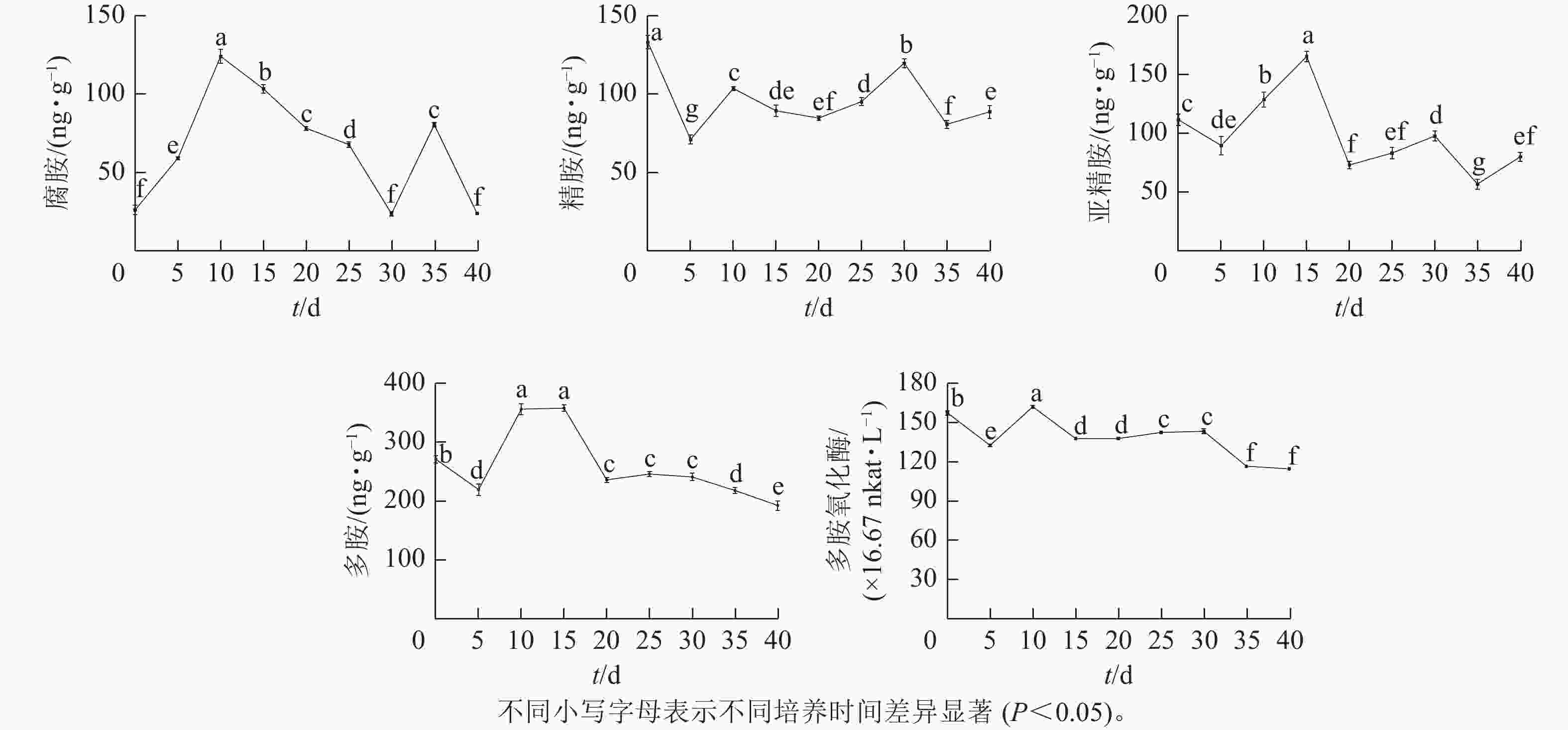
由图4可知:腐胺(Put)、精胺(Spm)和亚精胺(Spd)质量分数变化整体呈M型。腐胺在培养第10天和第35天时出现峰值。精胺在第10天和第30天时出现峰值。亚精胺在第15天和第30天时出现峰值。体胚发育的不同时期多胺质量分数差异显著(P<0.05)。
在球形胚形成时期(5~10 d),多胺(PAs)质量分数快速上升,球形胚发育至子叶胚期(10~30 d)时,腐胺质量分数下降;精胺质量分数在子叶胚期前呈下降趋势,至子叶胚时期呈上升趋势;球形胚时期,亚精胺质量分数逐渐上升,至心形胚、棒状胚时期急剧下降,子叶胚膨大、成熟时期亚精胺质量分数呈上升趋势。在子叶胚成熟后期(30~40 d),腐胺质量分数为急剧上升后下降,精胺和亚精胺质量分数为下降后上升。
腐胺、精胺和亚精胺质量分数变化共同决定多胺的变化趋势,多胺的峰回变化主要表现为愈伤组织增殖、体胚早期分化和子叶胚期成熟3个时期,多胺主要在体胚早期分化和发育阶段大量积累,说明多胺可能共同调控体胚的形成。
在体胚发生过程中多胺氧化酶活性呈M型变化趋势。在第10天和第30天出现峰值,在体胚分化早期(0~15 d)和子叶胚成熟后期(30~40 d),多胺氧化酶活性差异显著(P<0.05)。
在体胚分化早期(0~15 d),胚性愈伤组织增殖时,多胺氧化酶活性下降;球形胚形成时,多胺氧化酶活性快速上升呈明显的正相关作用;球形胚短暂增殖时,多胺氧化酶活性快速下降。在子叶胚期(20~40 d),子叶胚形成及成熟初期,多胺氧化酶活性又上升;在子叶胚后续膨大成熟时,多胺氧化酶活性急剧下降。
-
许多植物中同时存在内起源和外起源2种方式,如芍药Paeonia lactiflora[14]、香榧Torreya grandis ‘Merrillii’[15]。本研究石蒜品种‘梦幻少女’体胚发生为外起源,球形胚发生多为愈伤组织的外表层或亚外层,但不能完全排除内起源的可能。‘梦幻少女’体胚形态发育的过程经历了球形胚—心形胚—棒状胚—子叶胚,这与荔枝Litchi chinensis[16]、油棕Elaeis guineensis[17]、火炬松Pinus taeda[18]和白刺花Sophora davidii[19]等植物的体胚形态发生过程相似。
体胚与母体愈伤组织产生生理隔离是植株再生的重要前提,否则易出现胚畸形、退化、死亡等问题[20]。在荔枝、鹅掌楸Liriodendron chinense的早期原胚期[16, 21],以及枣Ziziphus jujuba[20]和苏玛栎Quercus shumardii[22]等的部分球形胚期均出现生理隔离形成的现象。本研究对‘梦幻少女’体胚发生过程的组织学特征观察发现:球形胚、心形胚形成维管束,与母体愈伤组织形成生理隔离。
-
在体胚发生过程中,淀粉、可溶性蛋白质、酶活性等的变化是相关基因表达和调控的结果[23]。在体胚发生过程中,作为能量物质,淀粉积累在细胞代谢、形态分化和发育等方面均起着重要的作用[23]。仙客来Cyclamen persicum在球形胚时期出现淀粉积累高峰[24]。人参Panax ginseng在成熟胚中淀粉积累量最高[25]。在本研究中,淀粉积累的高峰出现在‘梦幻少女’的球形胚时期,说明淀粉积累可能促进了球形胚的形成。
在体胚发生过程中,蛋白质同样是重要的物质和能量代谢基础。大蒜Allium sativum愈伤组织分化球形胚时,可溶性蛋白质质量浓度上升,在体胚后续发育至植株再生期间,可溶性蛋白质质量浓度不断下降,表明球形胚时期可溶性蛋白质的积累主要用于后续的发育和成熟阶段[26]。本研究中‘梦幻少女’体胚发生前期总蛋白质质量浓度保持稳定,在第25天时快速出现高峰,代谢活动加强,之后快速下降,表明迅速积累的总蛋白质可能为子叶胚形成和成熟提供了能量,该结果与橡胶树Hevea brasiliensis[27]、樱桃番茄Lycopersicon esculentum[28]等体胚发生研究的结果相一致。
POD、CAT和SOD为植物代谢中的重要酶类。POD可以促进木质素合成,与呼吸作用相关,进而推进体胚的形态发育[28];SOD作为一种保护酶和诱导酶,对胚胎发育具有促进作用,在体胚发育早期快速积累起到保护组织和细胞的作用[29];CAT具有提高抗逆性、提高抗氧化能力和延缓植物衰老的作用。本研究中POD、SOD活性的变化趋势与总蛋白质质量浓度相似,POD在体胚发生的早期变化不明显,在子叶胚形成时快速积累,说明POD的积累可能有利于子叶胚形成;SOD的峰值出现在培养的第5天和第20天,可能促进球形胚和子叶胚形态分化;CAT活性在培养第25天开始下降,此时子叶胚开始不断成熟。POD、SOD和CAT活性的整体变化趋势相似,说明子叶胚的成熟可能需要消耗多种抗氧化酶。
多胺的变化是影响体细胞胚发育的重要因素[30]。荔枝体胚中腐胺在球形胚时期发生了迅速的积累上升[16]。龙眼Dimocarpus longan愈伤组织分化为球形胚期间,腐胺、亚精胺和精胺均快速上升,其中腐胺变化占主导地位[31]。在本研究中,‘梦幻少女’球形胚时期,腐胺、亚精胺和精胺质量分数迅速提高,多胺的积累可能共同促进球形胚的分化。心形胚、棒状胚时期,腐胺、精胺和亚精胺质量分数都明显下降,说明子叶胚的形成需要同时消耗这3种物质。这与枸杞Lycium chinense[32]的研究结果相似,枸杞球形胚至成熟胚阶段,腐胺和精胺质量分数有所下降。在本研究的子叶胚初期,腐胺下降至谷底,精胺和亚精胺上升至新的峰值,此时多胺的变化可能共同促进体胚的成熟,多胺的变化趋势与龙眼[31]相似。多胺氧化酶与多胺中的亚精胺变化趋势相似,第10~15天和第30天出现峰值,体胚形态明显转化时发生明显的积累或消耗变化,这有利于球形胚形成和子叶胚成熟,多胺氧化酶活性变化趋势与荔枝[16]的相似。
-
在石蒜品种‘梦幻少女’体胚发生的0~40 d,体胚形态经历了原胚—球形胚—心形胚—棒状胚—子叶胚的过程。体胚发生过程中的生理特性变化显著,淀粉变化可能是球形胚形成、体胚形态发育与成熟的重要影响因素。在子叶胚形成及成熟时期,总蛋白质、过氧化酶活性和多胺变化可能发挥着相应的密切联合作用。多胺的积累和多胺氧化酶活性的提高可能促进体胚早期发育。在子叶胚形成及成熟时期,腐胺、精胺和亚精胺的变化相互发挥着密切的联合作用。
Cytological and physiological characteristics of somatic embryogenesis in Lycoris
doi: 10.11833/j.issn.2095-0756.20230321
- Received Date: 2023-05-22
- Accepted Date: 2023-08-07
- Rev Recd Date: 2023-07-12
- Publish Date: 2024-04-01
-
Key words:
- Lycoris /
- callus /
- somatic embryo /
- physiology and biochemistry
Abstract:
| Citation: | CHEN Yining, LEI Xue, LI Xin, et al. Cytological and physiological characteristics of somatic embryogenesis in Lycoris[J]. Journal of Zhejiang A&F University, 2024, 41(2): 243-251. DOI: 10.11833/j.issn.2095-0756.20230321 |










 DownLoad:
DownLoad: