-
光照是影响植物生长发育的重要环境因素之一,不仅提供了植物生长和生理活动所需要的能量,还作为一种信号调控植物的生长发育和形态建成[1]。因此,光是植物为了生长发育需要竞争的重要资源,特别是在生长密集的植物群落中。阳光中的红光(red light,R)和蓝光(blue light,B)被位于上层的叶片吸收用于光合作用,大部分的远红光(far red light,FR)被上层植物组织反射和传递给下层植被,导致红光与远红光比例(R/FR)下降、光合有效辐射(photosynthetically active radiation, PAR)减少。研究表明:下层植被感知到的R/FR可从全光照下的1.20降至0.05~0.70[2]。如拟南芥Arabidopsis thaliana单片叶遮盖可以将PAR从1 500 mmol·m−2·s−1减少到120 mmol·m−2·s−1,R/FR从1.20减少到0.20,且增加一片叶遮盖后PAR下降至40 mmol·m−2·s−1,R/FR下降至0.1[3]。
不同植物对光照的敏感程度不同。阳生植物在高光强条件下生长状况良好,但在遮阴或者弱光条件下生长不良,例如向日葵Helianthus annuus和月季Rosa chinensis等;而阴生植物则在长期遮阴条件下可以正常完成生活史,如三七Panax notoginseng和南方红豆杉Taxus chinensis var. mairei[4−5]。在遮阴条件下,阳生植物感知遮阴信号时会产生一系列避荫反应(shade avoidance response, SAR)以躲避低光,而阴生植物则通过产生一系列耐荫反应使植物获取更多光照来维系植株正常发育。前人对于阳生植物避荫反应的研究甚广,包括从下胚轴伸长、分枝减少[6]、比叶面积(specific leaf area,SLA)[7]增大和叶绿素a/b下降[8]等生理形态变化的研究,到生长素、赤霉素和油菜素甾醇与光信号通路互作共同调节植株避荫反应的分子机制,而对阴生植物耐荫机制的研究却十分匮乏,仅能借鉴个别阳生植物耐荫反应的研究。
本研究综述了荫蔽环境中阳生植物和阴生植物的生理形态、避荫反应和耐荫反应机制的研究进展,旨在为弱光环境下植物光响应机制尤其是阴生植物耐荫机制的研究提供参考依据,同时为提高植物光合效率,培育高产作物新品种,以及森林生态系统中林分调整、林下经济空间多层结构的搭建开辟有效路径。
-
植物可以通过感知光合有效辐射及光质的改变相应地调整其生长和发育策略,而阳生植物和阴生植物在遮阴条件下采取的策略大有不同。
-
在林冠层,许多阳生植物的光受体在感知到光强变化,主要是R/FR的降低,会出现一系列的避荫反应综合症,包括下胚轴和叶柄伸长、分枝减少和叶片偏下性,严重时还会导致分枝减少和叶面积增加,开花、结实提早[6, 9−10]。植物通过伸长下胚轴、叶柄或茎的长度来增加植物的高度[11],使照射到叶片的R/FR接近正常,以便获得更多可用的光照。研究表明:油菜Brassica napus和拟南芥在遮阴下会促进特定脂质的生物合成来增强下胚轴的伸长[12],同时拟南芥莲座叶叶柄也会伸长[13];由于附近植株的遮挡,光照强度较小,重要粮食作物大豆Glycine max在密植时产生了茎秆伸长等避荫反应特征,导致大豆倒伏,减产22%[14]。由于光合作用环境因素和光合作用产物积累的有限性,植物通过减少分枝来抵御弱光条件。拟南芥在低R/FR 时,constans-like 7 (COL7)抑制其分枝形成[15]。同时由于光照弱,叶片能获得的光照较少,植物通过增加比叶面积以提高获得光源的能力[7]。十字花科Brassicaceae 植物白芥Sinapis alba遮阴叶片比未遮阴叶片表现出较少的干物质和结构性碳水化合物的积累,比叶面积相对增加[16]。此外,由于林冠层下的红光和蓝光均减少,故吸收红光和蓝紫光的叶绿素a和叶绿素b质量分数显著增加,但叶绿素a/b有所下降[13]。
-
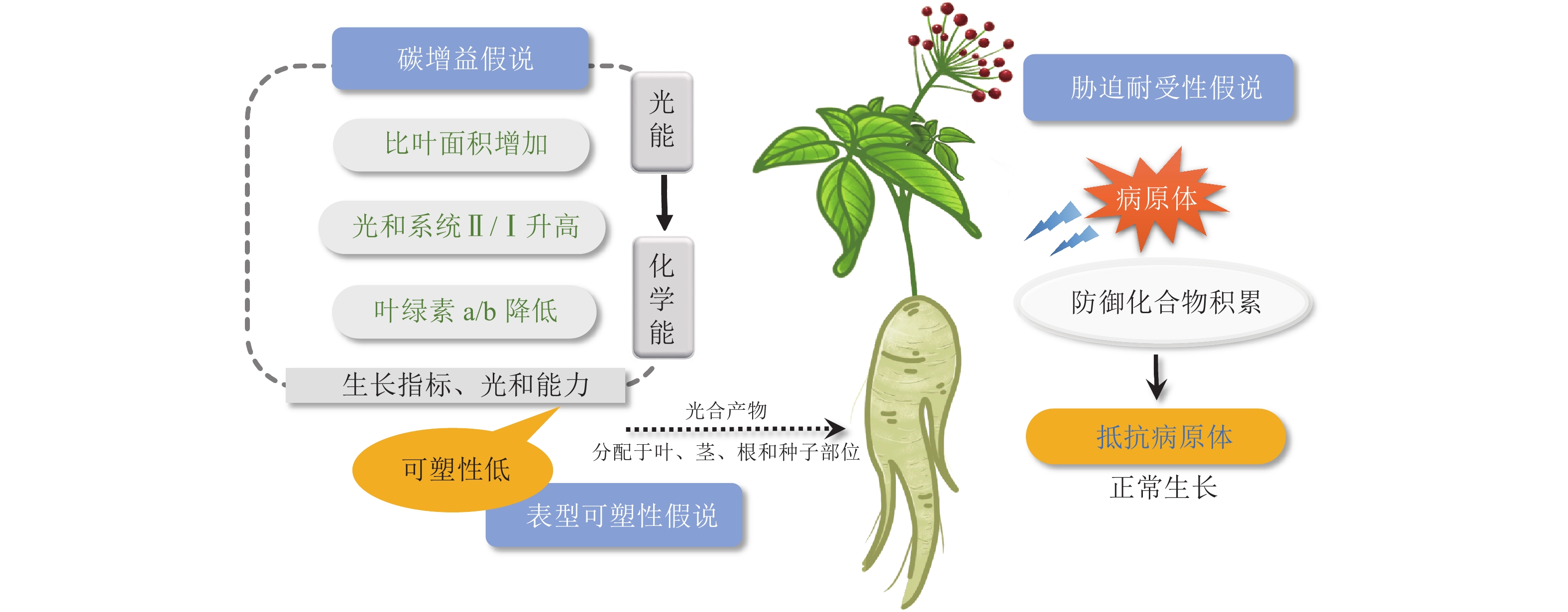
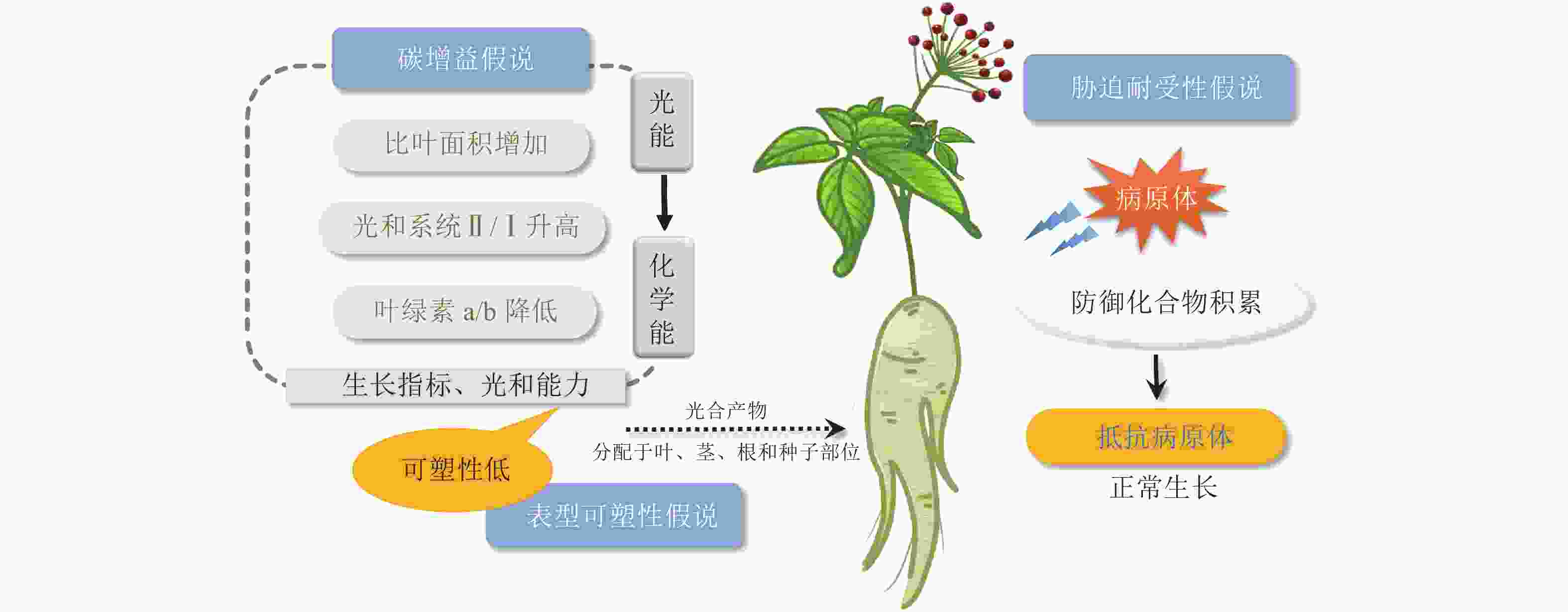
生长在林下的阴生植物发展出耐荫表型,以应对有限的光环境。关于阴生植物如何适应有限的光照,目前有碳增益、表型可塑性和胁迫耐受性3种假说[17]。
碳增益假说指出:植物能够提高光能利用效率的一切性状特征都可以增加碳的获得,减少呼吸成本,进而增强植物的耐荫性,阴生植物小到植物组织大到整株植物的范围内都有一些特征能促进碳的获得[18]。在叶片层次上,阴生植物通常具有更大的比叶面积以及更长的寿命。在光照强度低的情况下,阴生植物叶片利用较少的光能进行光合作用生成碳水化合物,具有较高的固定碳水化合物的能力。伴随着叶绿素b质量分数的升高,叶绿素a/b降低,以及光化学反应中心Ⅱ和Ⅰ的比例增加,阴生植物可将更多的光能转化为化学能,将碳水化合物存储在植株中[19]。光系统Ⅰ(PS Ⅰ)向光系统Ⅱ(PS Ⅱ)的转变是植物在较低的光照强度下最大限度地提高获取光能的一种方法[20]。研究发现:阴生植物华北蹄盖蕨Athyrium pachyphlebium和金线兰Anoectochilus roxburghii在降低光照时,PS Ⅱ反应中心的非光化学淬灭系数(non-photochemical quenching, NPQ)降低,PS Ⅰ和PS Ⅱ反应中心的光合电子传递速率以及PS Ⅱ反应中心的光化学淬灭系数变高,PS Ⅱ和PS Ⅰ比例增加,光能更高效地转化为化学能[19, 21]。同样,五加科Araliaceae阴生植物也进化出了适应度表型(PS Ⅱ的最大量子产量、低光饱和点和暗呼吸速率)来增加碳的获得,从而应对遮阴生境中的低光照环境[18]。
表型可塑性假说认为:阴生植物在不同光照条件下表现出较低程度的生理和形态可塑性。对木本耐荫物种的70多个性状进行Meta分析表明:木本耐荫植物相比阳生植物在大多数分析性状(如光合能力和生长指标)中具有低水平的可塑性[22]。因此,在遮阴环境下,阳生植物倾向于分配更多的资源来提高表型可塑性,产生避荫反应[18],而阴生植物并不会最大程度地生长,而是将更多的光合产物分配给植物的种子、叶、茎以及根等部位(图1),以便于植物在光照强度接近或低于整株植物光补偿点时维持正常生长状态[23−24]。
阴生植物在阴暗、温暖、潮湿的环境中生长,感染真菌病害概率大大增加。胁迫耐受性假说提出:对于阴生植物,最关键是要提高对生物和非生物胁迫的抵抗能力,而不是投入大量能量来促进植物的生长和发育[25]。代谢物作为植物与环境的适应产物,不仅是用于治疗病害的功效成分,还在植物体内参与信号传导、适应调节和机体防御等生物过程[26]。有研究表明:在遮阴环境下,植物的耐荫性及抵抗病原体和疾病的能力呈正相关[25],这与阴生植物在遮阴环境下防御化合物的高效积累有关。五加科人参属Panax三七和人参Panax ginseng是典型的阴生植物。目前已发现20多种影响五加科植物生长的叶面和根部病害[27],极大地威胁着三七和人参的质量和产量。在遭受病原体侵袭时,三七β-葡萄糖苷酶通过水解原人参二醇型人参皂苷,产生一系列具有更广谱、更强效抗真菌活性的稀有人参皂苷,确保三七在阴暗、温暖、潮湿环境中的生长发育(图1)。这在人参中也得到了证实[27]。
-
植物激素是一种植物自身代谢产生的物质,直接调控植物生长及发育[28]。阳生植物避荫反应相关的生理反应由光信号通路与许多植物激素相互作用来调节,其中主要包括生长素(Auxin)、赤霉素(GA)和油菜素内酯(BR)(图2)。
-
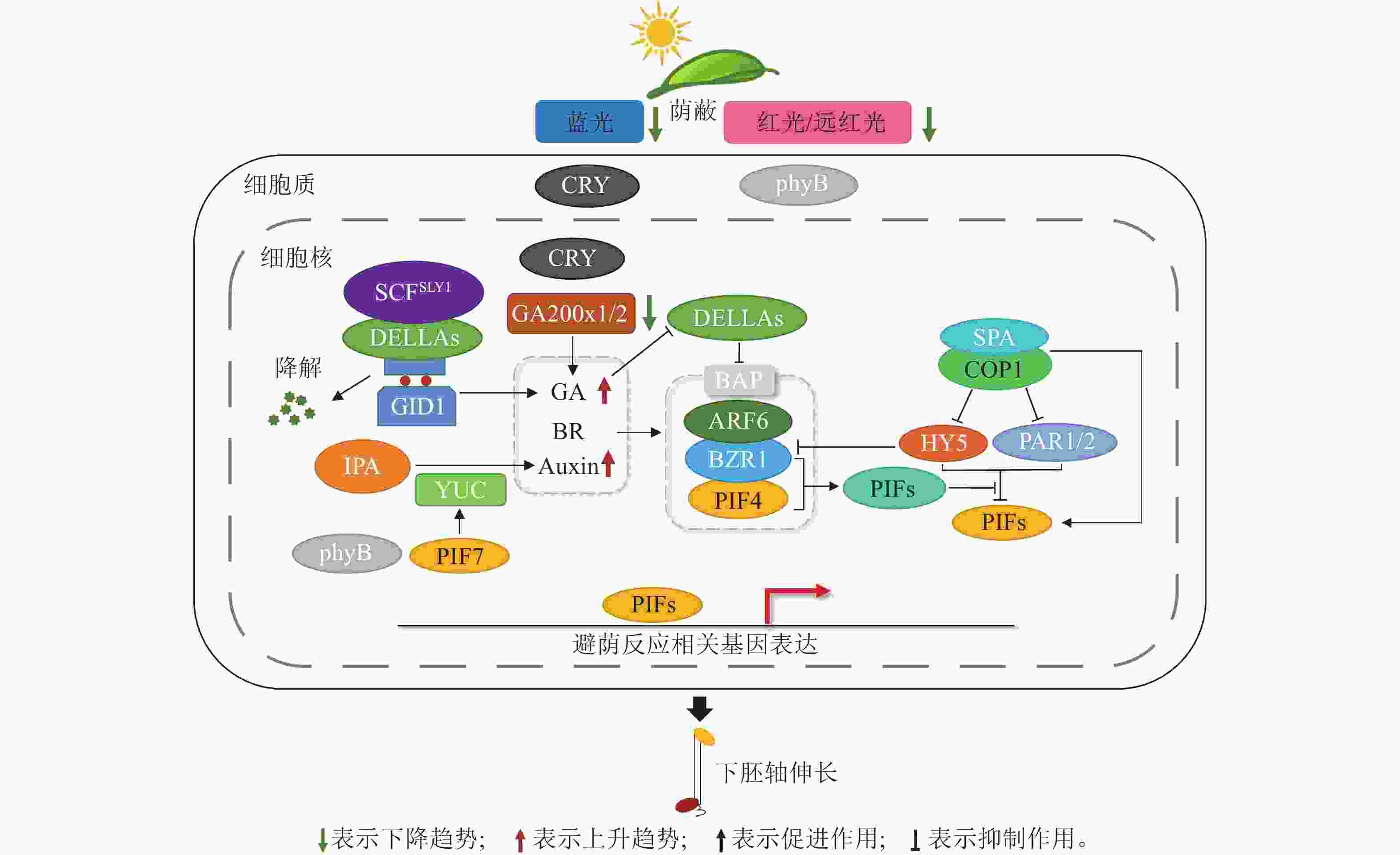
生长素对植物避荫反应中细胞伸长起主要调节作用。在幼苗中,它主要在子叶中产生,并输送到下胚轴和根中[29]。吲哚-3-乙酸(IAA)是主要的生长素,主要通过TAA1-YUC途径从色氨酸合成。色氨酸氨基转移酶(TAA1)将色氨酸转化为吲哚-3-丙酮酸(IPA),黄素单加氧酶(YUC)负责在限速步骤中将吲哚-3-丙酮酸转化为游离吲哚-3-乙酸[30]。遮阴条件下,低R/FR增强了生长素的响应能力,诱导生长素的生物合成[31]。在这个过程中,光敏色素互作因子(phytochrome interacting factor,PIF)发挥着关键作用。在遮阴环境下,盐过度敏感2 (salt overly sensitive2,SOS2)蛋白激酶能稳定PIF4和PIF5蛋白,而PIF7发生去磷酸化。它们共同作用,在生物合成、物质运输和信号传递过程中上调生长素活性。PIF4/5/7直接参与调控遮阴下黄素单加氧酶的表达。遮阴下PIF7去磷酸化导致黄素单加氧酶表达,使吲哚-3-乙酸增加(图2),随后通过生长素相关蛋白PIN3/4/7输送到下胚轴,促进植株伸长,发生避荫反应[32]。在正常光照条件下,光活化状态的phyB和CRY1能够与AUX/IAA蛋白相互作用,并抑制AUX/IAAs与生长素受体TIR1的结合,从而阻断生长素通路信号的传递[33]。
-
赤霉素是另一种促进避荫反应中茎和叶柄伸长的植物激素。Skp-cullin-F-box (SCF)E3泛素连接酶复合物由SLY1及SNE的F-box亚基组成,并与DELLA蛋白相互作用,以促进其泛素化和降解,并介导GA反应[34−35]。在遮阴条件下,蓝光减少,隐花色素CRY1处于非活跃状态,无法与GA通路中的赤霉素受体(GID1)或DELLA蛋白相互作用,赤霉素触发赤霉素受体与DELLA蛋白的相互作用。之后,SCFSLY1与DELLA蛋白结合,导致DELLA蛋白泛素化并降解[36],进一步将PIF从DELLA蛋白的抑制中释放出来(图2) ,并与避荫反应相关基因的启动子结合,促进植株伸长[36]。在大豆耐密植品种培育研究中,正常光照条件下,蓝光激活的CRY1增加了bZIP转录因子STF的积累,STF直接上调赤霉素氧化酶GmGA2oxs的转录活性,以降低赤霉素水平。遮阴下,蓝光减少抑制CRY1活性,造成STF蛋白丰度降低,GmGA2oxs的转录水平下降,赤霉素水平升高,促进下胚轴伸长[37]。Constitutive photomorphogenic 1 (COP1)是光形态建成的关键负调节因子,遮阴下R/FR降低,phyB失活促使DELLA降解,释放PIF。同时,COP1阻碍光信号通路上的bZIP转录因子家族的elongated hypocotyl 5(HY5)对避荫反应的抑制作用,促进下胚轴的伸长[38] (图2)。
-
油菜素内酯在避荫反应中也发挥了重要作用。油菜素内酯信号通路激活后促进了油菜素内酯信号转录因子(BZR1)的去磷酸化,然后进入细胞核并促进油菜素内酯合成抑制剂(BRZ)下游基因的表达[39]。研究表明:BES1/BZR1通过直接和PIF4相互作用对下游目标基因起到调节作用(图2) ,促进植物下胚轴伸长,产生避荫反应[40]。此外,COP1也参与了油菜素内酯介导的避荫反应,比如COP1能在黑暗中稳定PIF1、PIF3、PIF4和PIF5 [41−42],COP1/SPA复合物能够抑制糖原合成酶激酶(BIN2)引发的PIF3磷酸化,从而与PIF3相结合并稳定PIF3[43],增强植物的避荫反应(图2)。
-
避荫反应的发生常常是几种激素和光信号通路共同作用的结果。油菜素内酯信号调控因子BZR1与生长素响应因子6 (ARF6)和PIF4一起形成了BAP调节模块(图2)。遮阴条件下,此模块被激活来响应各种生长调控信号[44−45]。BZR1可以直接和生长素生物合成基因PIF4的启动子结合,油菜素内酯和生长素信号通路通过关键的BAP模块相互连接促进避荫反应[46]。DELLA蛋白是BAP调节模块的一个重要负调控因子,在赤霉素的作用下迅速降解[47]。遮阴下phyB受到远红光抑制并同时降低PIF蛋白活性,DELLA蛋白通过阻止PIF4和BZR1与其DNA靶点结合及与ARF6结合,触发生长素介导的避荫反应[39](图2) 。Paclobutrazol resistance 1 (PRE1)是BZR1和PIF4共同的下游基因,该基因由油菜素内酯、赤霉素和生长素共同调节[38],它们通过和蛋白激酶受体(protease-activated receptor 1, PAR1)形成异二聚体,阻碍PAR1/2及HY5对PIF4的抑制作用[48],维持PIF蛋白活性,发生避荫反应(图2)。然而,上述3种激素并不是同步对避荫反应进行调控的。对遮阴处理早期拟南芥下胚轴的生长研究表明:生长素和油菜素内酯出现的时间比赤霉素早,基因表达的变化与表型联系更为紧密[49]。
-
目前对于耐荫反应的机制研究还相对匮乏。结合耐荫反应分子机制最新研究进展并借鉴阳生植物避荫反应机制,推测阴生植物可能通过抑制避荫反应和增强耐荫反应双重复杂机制来保证荫蔽环境中的正常发育。
-
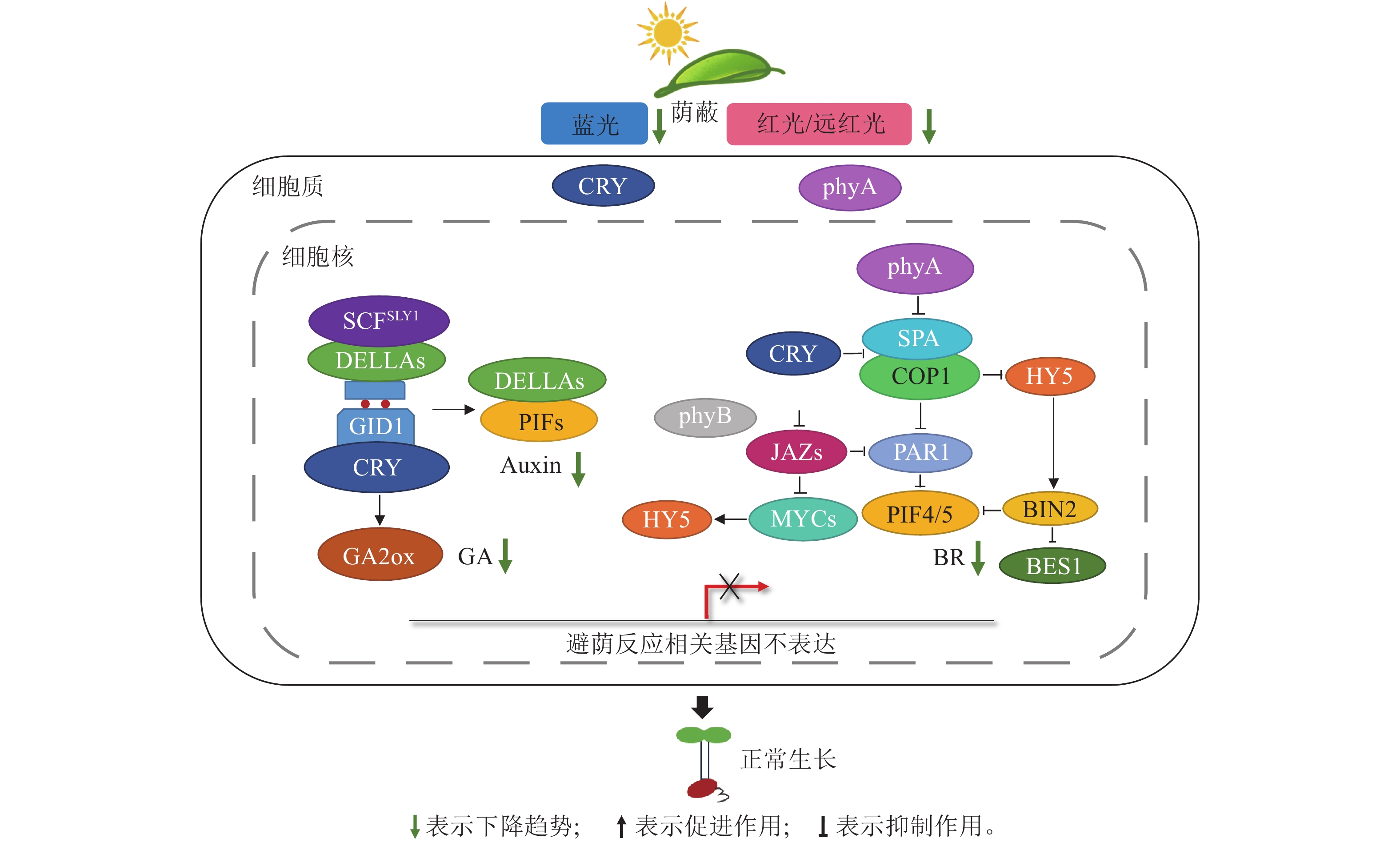
光敏色素phyA是一类典型的阳生植物避荫反应拮抗因子,在抑制阴生植物避荫反应的过程中发挥重要作用。phyA在高远红光下发挥作用[50],可以抵御由光敏色素phyB介导的植物伸长[2, 51]。在强遮阴下(R/FR为0.1),野生型拟南芥phyA被显著激活,但其下胚轴并未伸长;相应地,phyA突变体下胚轴出现过度伸长,而COP1的突变完全抑制了其下胚轴的伸长。这表明强遮阴下,拟南芥phyA以COP1依赖的方式抑制避荫反应 [52]。phyA介导的光信号通路还可以通过与油菜素信号通路互作的方式促进耐荫反应[53]。近期研究发现:强遮阴条件下(R/FR为0.1),拟南芥表现出耐荫反应。活跃状态下的phyA会减少COP1的核定位,导致其下游靶基因HY5表达量升高。HY5可以促进蛋白激酶BIN2的活性,从而使BES1磷酸化,抑制油菜素内酯信号通路,从而抑制下胚轴伸长(图3) 。同时,在强遮阴下COP1会通过干扰BIN2和PIF相互作用而稳定PIF[43]。phyA通过减少COP1核定位,导致COP1/SPA1复合体不能影响BIN2和PIF的互作,PIF4/5发生磷酸化降解,其靶基因油菜素内酯合成基因不能被激活,进一步导致油菜素内酯通路下游与下胚轴伸长相关的木葡聚糖内转葡糖糖基酶/水解酶(XTH)基因表达降低,避荫反应减弱[52]。因此,推测阴生植物的耐荫机制可能与强遮阴下拟南芥的耐荫机制类似,通过phyA-COP1依赖的方式与油菜素内酯通路互作来调控。
另一类拮抗因子是bHLH及bZIP类转录蛋白。在遮阴环境下,植物对病原菌和植食性昆虫的敏感性增强,茉莉酸(JA)通路被诱导,MYC转录因子上调表达,并与HY5直接相互作用,增强其转录活性和HY5蛋白稳定性,从而抑制下胚轴伸长;另外,HY5还可以与BZR1的活性形式直接相互作用,并减弱其转录活性[54],抑制避荫反应。同时,茉莉酸信号转录抑制因子JAZ (jasmonate ZIM-domain)能够与PAR1/PAR2结合并抑制其表达。茉莉酸的增加导致JAZ蛋白的降解,PAR1/2被释放[55],PAR1/2通过与PIF形成异源二聚体来阻止PIF激活下游基因的表达, 进而抑制荫蔽环境下植物茎干及叶柄等的过度伸长[56]。
近期研究证实:上述2种避荫反应拮抗因子共同作用参与遮阴环境下植物的耐荫反应。强遮阴下拟南芥phyA促进耐荫反应的发生,PAR1/2会在COP1下游直接影响植物耐荫反应(图3) 。COP1作为E3泛素连接酶发挥作用,介导SAR负调节因子PAR1和HY5的泛素化和降解。强遮阴下,phyA抑制COP1入核,PAR1和HY5蛋白积累,抑制PIF4的表达,发生耐荫反应[38, 57]。
-
蓝光受体CRY参与植物生长和发育调节,CRY1和CRY2在蓝光下与SPA蛋白相互作用,以抑制COP1/SPA活性,稳定HY5,抑制植株伸长[6](图3)。研究证明:在蓝光环境中蕨类植物的CRY (5个成员)发生了基因扩增现象,其植株叶片叶绿体更多,对蓝光和二氧化碳不敏感,气孔不会主动关闭,植物通过产生活性氧而让叶片气孔快速打开,使其在低光条件下也能进行高效的光合作用[58−59]。在大豆中,GmCRY1b过表达植株耐荫性显著提高,实现了密植条件下的高产[14]。因此,阴生植物可能通过CRY的扩增或提高CRY的转录活性来增强遮阴环境下的光能利用率。基于上述理论,推测在遮阴下,蓝光减少,但阴生植物可能存在高敏感性CRY家族基因,在低蓝光下仍处于活跃状态,进而调节GA2ox基因的上调表达导致赤霉素减少,同时CRY与DELLA结合,DELLA蛋白积累并且与PIF结合导致生长素减少(图3),抑制植株伸长[36],从而使阴生植物在遮阴环境下产生耐荫反应,维持正常生长。
-
由于阴生植物与阳生植物在不同光照强度的环境中生长,故2类植物在相同的遮阴环境下会产生不同的变化。阳生植物在遮阴条件下产生的避荫反应依赖于复杂的避荫机制。在遮阴条件下,红光减少,远红光增加,R/FR下降,蓝光减少,光敏色素和隐花色素均可通过促进植株伸长等,使植株适应因遮阴带来的生理和形态变化。阴生植物则生活在光照强度较弱的林下,叶绿素a/b较低[60],相较于阳生植物有更大的比叶面积,可以最大程度地利用光能进行光合作用,维持植株正常生长并且将碳能储存在植株中。此外,阴生植物在阴暗潮湿的林下也会积累更多次生代谢物来抵御环境带来的病害。但阳生植物在过度遮阴的情况下,下胚轴不会伸长,出现耐荫的性状,故推测在遮阴情况下,阴生植物的耐荫分子机制与阳生植物强遮阴下的反应类似,低R/FR下,phyA减少COP1的核定位和JAZ蛋白的降解致使PAR1和HY5蛋白积累,进而BIN2和PAR1抑制PIF蛋白活性,抑制植物伸长;同时低蓝光下有活跃状态的CRY调节GA2ox基因的上调导致赤霉素减少,并且积累的DELLA与PIF结合导致生长素减少,植株避荫反应基因不表达,出现耐荫性状。
总之,对阳生植物避荫反应研究较多,对分子机制的了解也更为透彻,对阴生植物耐荫机制的深入研究较少。在农业生产中,合理密植和间套作是提高作物单产的重要手段。然而,对于主要栽培作物,这2种栽培模式下光合能力减弱和光质的改变导致光合作用减弱和碳水化合物的合成减少,产量潜力受到限制,从而引起不利的避荫反应综合症,造成作物大幅减产。在此情况下,研究耐荫反应机制对培育高光效率和高产作物新品种(系)具有重要意义。因此,后续需要筛选适合的阴生模式植物并深入研究其光合作用及光信号转导分子机制,阐明阴生植物光能高效利用的调控网络,并利用分子设计育种等策略,尝试改良作物主栽品种,创制高光效种质资源。另外,阴生植物长期生长在病原菌繁多的阴暗潮湿的环境中,在保证自身生长的同时还进化出了出色的防御机制,深入解析阴生植物协同适应遮阴和生物胁迫的分子机制,有助于进一步理解植物环境适应性和发育权衡的调控机制,为培育抗逆、高产的作物新品种提供理论依据和基因靶点。
在森林生态系统中,林分结构影响林木的生长和木材的品质。研究表明:在遮阴环境下耐荫性和木材的密度呈正相关[61]。一些生活在下木层和灌木层的阳生小乔木无法与林冠层的高大乔木竞争光照,因此获得的光能较少,又因其耐荫性差,难以利用下木层和灌木层空间,木材密度较小,经济效益低;而阴生树种的耐荫性能较好,木材密度大,但是生长缓慢,因此短期经济价值较小。所以除了通过阳生树种和阴生树种间作来获得短期和长期的收益外,也可以探寻阳生树种的耐荫性,以期达到既不影响其生长发育又使其木材密度最大化的目的。在种植上层林木的同时,利用林冠层下的林荫及林下土地资源种植阴生植物,不仅有利于调整林分结构,丰富生物多样性,更有助于提高粮食作物和药材的产量,大幅度提升经济效益。近年来,林下经济发展迅速,已成为山区经济发展的优势产业、种植业结构调整的特色产业、农民脱贫致富的支柱产业和大众创业的新兴产业。因此,进一步深入研究植物的弱光响应机制,为林下种植模式中空间多层结构的搭建提供理论指导,对提高森林能量转换率和资源利用率具有重要意义。
Research progress on plant physiological morphology and light response mechanism in shaded environments
doi: 10.11833/j.issn.2095-0756.20240187
- Received Date: 2024-02-26
- Accepted Date: 2024-07-11
- Rev Recd Date: 2024-07-03
- Available Online: 2024-08-27
- Publish Date: 2024-11-20
-
Key words:
- shaded environments /
- shade tolerance response /
- shade avoidance syndrome /
- plant hormones /
- light signaling pathways
Abstract: Light is a crucial environmental factor affecting plant growth and development. It is of great scientific significance and application value to enhance plant yield and quality in agricultural production by improving its photosynthetic efficiency. In dense plant communities, lower plants receive less light energy due to the coverage of upper vegetation, so lower plants need to compete for more light energy to maintain growth. Plants have two strategies to obtain more light energy: shade avoidance syndrome (SAS) and shade tolerance response (STR). Research on SAS is relatively thorough, but there is a lack of in-depth research on STR. This paper provides an overview of how sunny plants adapt to lower light level by extending hypocotyl, petioles, stems and other physiological morphological changes in shaded environments. At the same time, shade tolerant plants respond to limited light conditions by exhibiting shade resistance characteristics such as promoting carbon acquisition, low phenotypic plasticity ability and improving stress resistance. Combined with the mechanism of shade avoidance response of sunny plants in response to low light environment through the interaction between hormones and light signaling pathways, the shade tolerance response mechanism of shade tolerant plants in shaded environments is studied, which involves both activating antagonistic factors to inhibit shade avoidance syndrome and improving the transcription activity of shade tolerance response genes to enhance low light adaptability. This review provides reference for research on the mechanism of different plants responding to low light environments, and proposes effective ways to improve the efficiency of plant light energy utilization, cultivate crop varieties with high light efficiency, and construct efficient forest ecosystems. [Ch, 3 fig. 61 ref.]
| Citation: | LIU Pei, WU Yufen, WANG Xiaofeng, et al. Research progress on plant physiological morphology and light response mechanism in shaded environments[J]. Journal of Zhejiang A&F University, 2024, 41(6): 1313-1322. DOI: 10.11833/j.issn.2095-0756.20240187 |










 DownLoad:
DownLoad: