-
铅(Pb)是常见的土壤重金属污染源之一,具有不可降解性、生物累积性和高毒性,易进入食物链,最终损害人类健康[1]。植物修复是经济、环保的修复铅污染土壤的方法[2],其中,观赏植物比食用植物更具优势[3−4]。与草本观赏植物相比,木本观赏植物由于其高生物量以及发达的根系在植物修复中更具潜力[5]。八仙花Hydrangea macrophylla是常见的木本观赏植物,世界各地均有栽培。八仙花因其生长迅速、适应性强、观赏价值高等优点,广泛应用于城市园林造景、盆花栽培及鲜切花生产[6]。此外,八仙花对铅有极强的耐性,是极具修复潜力的植物[7−8]。
目前,已有的相关研究多从生长生理、重金属积累特性、组学分析角度探讨八仙花对铅的耐受差异及机制[7−12],但针对八仙花根际环境特征对其耐铅机制的影响研究未见报道。根际包含大量复杂生物及生态过程,是生态系统中最活跃的交互界面之一[13] 。重金属胁迫不仅直接影响根系生长,还会诱导植物根系分泌各种化合物,这些根系分泌物会改变土壤理化性质,影响土壤酶活性,改变根际铅的化学形态,进而影响植物对重金属的耐性及吸收[14−15] 。然而,重金属胁迫下植物根系氧化应激、根系分泌物和主要根际特征的综合响应机制鲜有研究。
本研究以八仙花为材料,研究其在不同质量分数铅处理下的铅吸收特性、根系氧化应激、根系分泌物及主要根际特征等,探讨其对重金属铅的吸收和耐受机制,为提高八仙花对铅污染土壤的修复效率和园林植物修复重金属污染土壤的应用提供参考。
-
供试土壤采自陕西省杨凌区农田0~20 cm表层土,其理化性质为:pH 7.44、有机质14.76 g·kg−1、全氮112 mg·kg−1、有效磷7.80 mg·kg−1、速效钾219 mg·kg−1、铅26.50 mg·kg−1。在风干过筛后的土壤中加入Pb(NO3)2溶液,老化4个月后得到铅质量分数为500、1 500、2 500 mg·kg−1的污染土壤。土壤实际铅质量分数分别为529.10、1 532.27、2 524.98 mg·kg−1。土壤铅梯度设置参考GB 36600—2018《土壤环境质量 建设用地土壤污染风险管控标准》及预试验结果。
选取健康、长势一致的1年生八仙花扦插苗(株高16~18 cm)栽植于塑料盆(18 cm×16 cm×11 cm)中,每盆2 kg土,处理组土壤铅为500、1 500、2 500 mg·kg−1,对照为不添加铅的洁净土壤,每盆1株,重复3次。试验于西北农林科技大学科研温室中进行,光照为自然光,室温保持在 20~25 ℃,空气相对湿度为65%。试验期间定期浇水,且未施用植保产品。50 d后收获分析。
-
试验结束后拍照记录植物样本。采用根系扫描仪系统(LA-S,万深)测定根系形态参数。将洗净的植物组织置于烘箱中,105 ℃下杀青30 min,70 ℃烘干至恒量,记录干质量。土壤样本经自然风干、粉碎、过2 mm筛后,用微波消解仪(MA165-001, Milestone)进行消化[16],采用火焰原子吸收光谱仪(PinAAcle 900F, PerkinElmer)测定铅。利用植物标准品GBW-07603(GSV-2)和土壤标准品GBW-07405(GSS-5)进行质量控制,回收率分别为102.3%和102.1%。生物富集系数(BCF)=植物铅质量分数/土壤剩余铅质量分数;转运系数(TF)=植物地上部铅质量分数/植物地下部铅质量分数[17],耐受指数=铅胁迫组根长/对照组根长×100%[18]。耐受指数可用于判定植物对重金属的耐受程度,高于50%时说明植物对重金属有较强的耐受性。
-
相对电导率(EC)和过氧化氢(H2O2)依据参考文献[19−20]的方法测定。超氧化物歧化酶(SOD)、过氧化物酶(POD)和抗坏血酸过氧化物酶(APX)活性参照参考文献[17, 21]的方法测定。抗坏血酸(AsA)和谷胱甘肽(GSH)参照文献[22]的方法测定。可溶性糖(SS)和脯氨酸(Pro)依照文献[20]的方法测定。
-
将植物根系洗净擦干后放于盛有250 mL超纯水的烧杯中,杯身用锡箔纸覆盖以避光,室温下收集6 h,过滤后得到根系分泌物[23]。根系分泌物提取与测定参照JIN等[12] 的方法。通过GC-MS分析根系分泌物的组成。采用Rtx-5MS毛细管柱(30 m × 0.25 mm内径, 0.25 μm膜厚)分离根系分泌物。自动进样器在30∶1模式下,进样1 μL衍生化样品。进样口温度为280 ℃,离子源温度为230 ℃,传输线温度为320 ℃。升温程序从50 ℃开始,保持0.5 min,然后以15 ℃·min−1升至320 ℃,保持9 min。全扫描质谱范围为35~650 m·z−1。采用NIST17数据库对代谢产物进行鉴定。
-
土壤pH值由pH计测定,土壤EC值由电导率仪测定,用重铬酸钾容量法测定土壤有机质[24] 。过氧化氢酶活性、脲酶活性和蔗糖酶活性测定参考文献[25]的方法。根际土壤不同铅形态采用Tessier连续提取法[18] 提取,再用火焰原子吸收光谱仪测定其质量分数。
-
数据为3个平行独立试验的平均值±标准差。利用SPSS 26软件进行单因素方差分析(One-way ANOVA)和LSD多重比较(P<0.05)。用Origin 2019b软件绘图,Word 2021制表。
-
如图1所示:八仙花在所有处理下均能存活,即使在2 500 mg·kg−1铅处理下,八仙花也未出现叶片发黄、失水萎蔫甚至整株干枯死亡等重金属毒害症状,但是株高却明显下降。由表1可见:八仙花生物量、根系总长、根系表面积和根尖数随着铅胁迫质量分数的增加而显著降低(P<0.05,表1),2 500 mg·kg−1铅处理下均达到最低,并伴随着根系颜色变褐。耐受指数均大于50%,说明八仙花对铅具有较强的耐受能力。
铅质量分数/ (mg·kg−1) 生物量/ (g·株−1) 根系总长/cm 根总表面积/cm2 根尖数/个 耐受指数/% 0(ck) 31.14±0.39 a 5 395.37±211.58 a 3 444.48±115.29 a 8 260.62±392.67 a 100.00 a 500 26.10±0.08 b 3 800.38±136.66 b 2 346.04±74.01 b 4 741.27±80.97 b 72.22±0.46 b 1 500 25.17±0.06 c 2 658.66±150.63 c 1 610.68±52.75 c 3 784.25±151.52 c 66.09±0.74 c 2 500 23.77±0.47 d 2 204.41±79.11 d 1 432.78±91.62 d 3 207.75±95.11 d 56.41±0.43 d 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 Table 1. The growth parameters of H. macrophylla under Pb stress
-
随着土壤铅质量分数的增加,八仙花富集系数、转运系数呈下降趋势,地上及地下部铅质量分数呈上升趋势(表2)。各铅处理的地下部铅质量分数均高于地上部铅质量分数,表明八仙花主要将铅固定在根部。随着土壤铅质量分数的增加,富集系数较对照分别显著降低了27.1%、50.0%和58.3% (P<0.05)。
铅质量分数/(mg·kg−1) 地上部铅/(mg·kg−1) 地下部铅/(mg·kg−1) 富集系数 转运系数 0(ck) 2.44±0.06 d 6.24±0.12 d 0.48±0.06 a 0.39±0.02 a 500 14.25±0.13 c 121.33±0.22 c 0.35±0.05 b 0.12±0.01 b 1 500 29.83±0.63 b 256.83±0.38 b 0.24±0.01 c 0.12±0.01 b 2 500 36.00±0.50 a 448.38±1.88 a 0.20±0.01 d 0.10±0.01 c 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 Table 2. Pb concentration, enrichment coefficient and transport coefficient of H. macrophylla under Pb stress
-
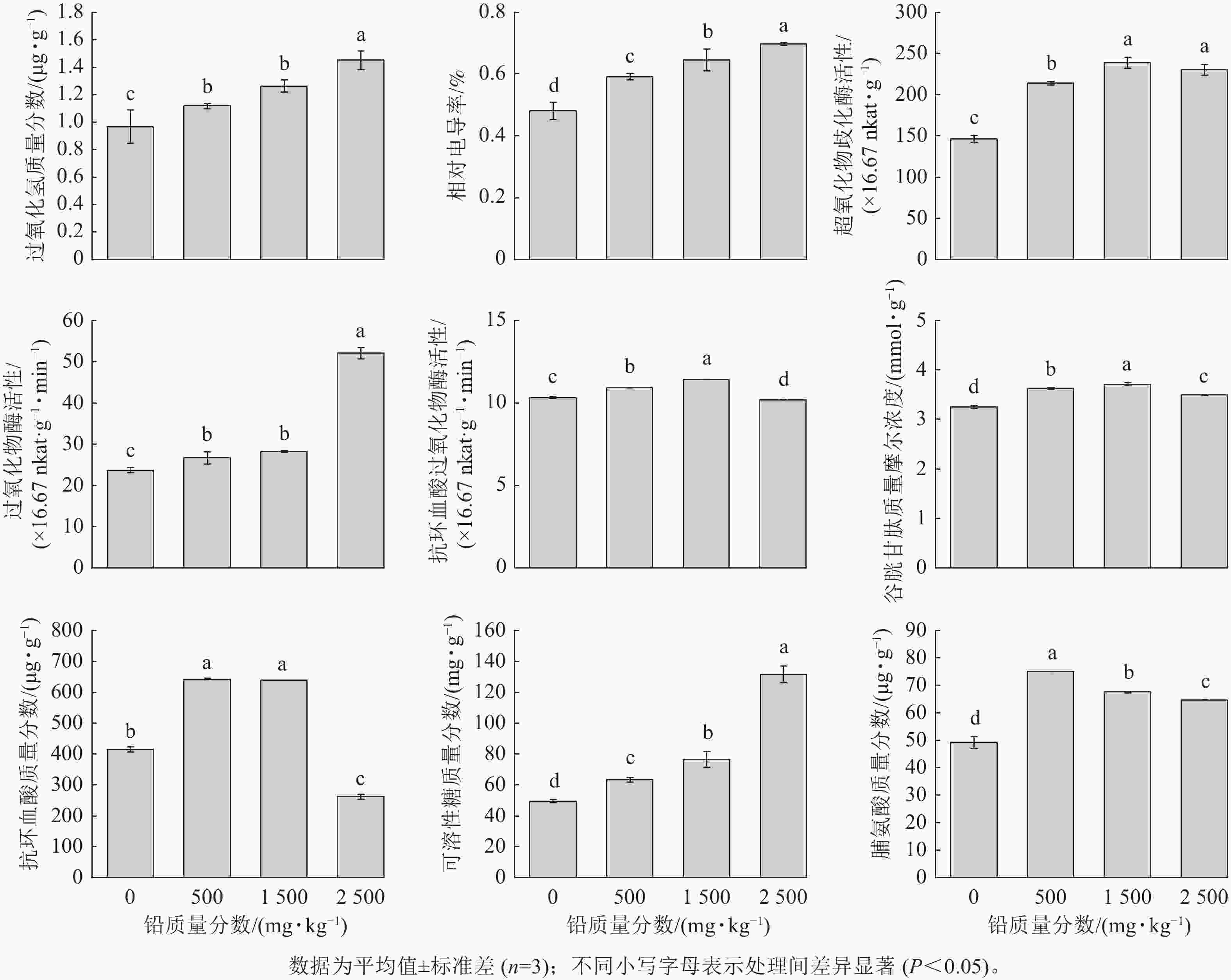
由图2可见,随着铅胁迫质量分数的增加,八仙花根系H2O2质量分数及相对电导率均逐渐上升。表明铅胁迫促使植物体内H2O2积累,对细胞膜造成氧化损伤,使得电解质渗漏增加。八仙花根系中SOD、APX活性及GSH质量摩尔浓度、AsA质量分数随铅质量分数的升高呈先升高后下降的趋势,POD活性随铅质量分数的升高而升高。其中SOD、APX活性及GSH质量摩尔浓度在1 500 mg·kg−1铅处理时最高。AsA质量分数在500、1 500 mg·kg−1铅处理下显著高于对照(P<0.05)。POD活性在2 500 mg·kg−1铅处理时最高,为对照的2.2倍。随着铅胁迫质量分数的增加,可溶性糖质量分数逐渐升高,而游离脯氨酸质量分数则呈先升高后下降的趋势,500 mg·kg−1铅处理时脯氨酸质量分数达到最高。
-
由表3可见:共检测到八仙花根系分泌物249种,其中相对含量最多的分泌物为脂肪酸,最少的为氨基酸。1 500 mg·kg−1铅处理分泌物数量最多(78种)。铅胁迫下八仙花根系分泌脂肪酸相对含量较对照显著上升(P<0.05);有机酸相对含量随铅质量分数的增长呈先上升后降低的趋势,在500 mg·kg−1铅处理下达到最高;氨基酸相对含量在2 500 mg·kg−1铅处理下最高,500、1 500 mg·kg−1铅处理下显著降低(P<0.05);铅胁迫下碳水化合物相对含量显著低于对照(P<0.05);各铅胁迫处理下胺类相对含量显著高于对照(P<0.05);醇类除了1 500 mg·kg−1铅处理下是对照组的69.7%,其他处理下变化不显著。
铅质量分数/
(mg·kg−1)分泌物
数量相对含量/% 脂肪酸 有机酸 氨基酸 碳水化合物 醇类 酯类 胺类 其他 0(ck) 73 25.62±0.69 b 12.33±0.47 b 2.79±0.10 b 9.54±0.06 a 19.38±0.09 a 12.77±0.54 a 6.34±0.30 c 11.23±0.44 a 500 52 25.50±0.32 b 17.28±0.12 a 1.76±0.04 c 8.40±0.10 c 19.82±0.57 a 8.08±0.18 c 13.83±0.65 b 5.68±0.03 c 1 500 78 26.13±0.74 a 11.17±0.51 c 0.85±0.04 d 8.93±0.10 b 13.50±0.13 b 12.32±0.56 a 18.10±0.72 a 8.97±0.75 b 2 500 46 26.83±0.33 a 9.52±0.37 d 6.44±0.48 a 8.95±0.04 b 19.05±0.88 a 9.60±0.34 b 13.43±0.28 b 5.51±0.53 c 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 Table 3. Effect of Pb stress on the quantity and relative content of root exudates of H. macrophylla
-
由表4可见:500 mg·kg−1铅处理下土壤pH较对照无显著变化,1 500、2 500 mg·kg−1铅处理下pH显著降低(P<0.05)。土壤EC在500 mg·kg−1铅处理下显著下降(P<0.05),在1 500、2 500 mg·kg−1铅处理下显著上升(P<0.05)。处理组土壤有机质质量分数较对照组分别显著增加了18.6%、18.6%、25.8% (P<0.05)。土壤蔗糖酶和过氧化氢酶活性随土壤铅质量分数的增加而增加,2 500 mg·kg−1铅处理下活性最高。土壤脲酶活性随土壤铅质量分数增加呈先上升后下降的趋势,其中1 500 mg·kg−1铅处理下活性最高,为对照的1.3倍。对照组土壤中残渣态、有机物结合态铅质量分数及占比均达到最大,而500、1 500、2 500 mg·kg−1铅处理下,铁锰氧化物结合态铅质量分数及占比最大。除有机物结合态铅在500 mg·kg−1铅处理下占比与对照相比上升外,残渣态和有机物结合态铅占比随着铅质量分数的增加而呈降低趋势,可交换离子态、碳酸盐结合态铅呈相反趋势。
铅质量分数/
(mg·kg−1)pH 电导率/
(μS·cm−1)有机质/
(g·kg−1)蔗糖酶活性/
(mg·g−1·d−1)脲酶活性/
(mg·g−1·d−1)过氧化氢酶活性/
(mg·g−1·20 min−1)0(ck) 8.16±0.13 a 126.10±5.58 c 2.85±0.03 c 0.21±0.03 d 114.00±0.82 c 2.01±0.01 d 500 8.21±0.17 a 92.33±3.17 d 3.50±0.27 b 0.30±0.02 c 143.62±1.45 b 2.16±0.05 c 1 500 7.59±0.20 b 173.43±6.92 b 3.50±0.04 b 1.94±0.06 b 151.52±1.40 a 2.44±0.03 b 2 500 7.73±0.15 b 260.33±6.92 a 3.84±0.02 a 2.24±0.04 a 112.91±0.68 c 2.54±0.02 a Table 4. Effect of Pb stress on the main rhizosphere characteristics of H. macrophylla
铅质量分数/
(mg·kg−1)铅化学形态质量分数及百分比/ (mg·kg−1) 可交换态铅 碳酸盐结合态铅 铁锰氧化物结合态铅 有机物结合态铅 残渣态铅 0(ck) 0 0.13±0.15 (0.53%) 0 8.96±1.36 (35.78%) 15.95±0.14 (63.69%) 500 1.43±0.12 (0.28%) 9.53±0.31 (1.84%) 274.67±12.22 (52.91%) 199.84±5.20 (38.50%) 33.63±3.71 (6.48%) 1 500 17.37±1.63 (1.17%) 74.27±3.79 (5.01%) 933.33±16.65 (62.96%) 416.48±3.39 (28.10%) 40.88±0.88 (2.76%) 2 500 52.67±10.40 (2.43%) 122.35±22.13 (5.66%) 1413.33 ±196.34 (65.34%)524.64 ±13.35 (24.25%)50.08±16.79 (2.32%) 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 -
重金属胁迫对植物生长存在一定的剂量效应,吴桐等[26]发现随着铅质量分数的升高,八仙花的地上干质量呈先增加后下降的趋势。本研究不同质量分数铅胁迫均抑制了八仙花的生长。这是因为重金属胁迫促使植物激活自身防御系统,促进抗氧化酶及抗氧化物质的合成,从而清除体内过量的活性氧,而这一过程需消耗大量能量,故而影响植物的正常生长[27]。重金属胁迫还会阻碍植物根系发育,降低根系发达程度及根系表面活性位点数量,减弱植物从环境中吸收养分和重金属的能力[28]。八仙花对铅有较强的耐受性,通过根部细胞壁吸附和液泡区室化作用,以及膜蛋白严格限制转运,阻断铅向八仙花地上部的流动[11, 29],将铅储存在根部以适应铅污染土壤,提高自身对铅的耐性。
-
H2O2是一种重要的活性氧,反映植物氧化应激水平[30]。外源铅胁迫促使八仙花根系细胞产生过量的H2O2,对细胞膜造成氧化损伤,破坏细胞膜完整性。重金属离子可与细胞膜蛋白、巯基及磷脂类物质结合,使得细胞膜结构损伤、选择性降低及通透性增加,大量电解质渗向细胞外,重金属离子进入胞内,导致细胞内外离子失衡及细胞代谢功能紊乱[31]。
SOD、POD、APX、GSH和AsA是抗氧化系统中主要的抗氧化酶和非酶抗氧化剂,可以有效去除植物体内过量H2O2[32−33],降低重金属对植物的氧化损伤[34]。本研究中铅胁迫下POD活性提高, SOD、APX活性及GSH、AsA水平呈现先上升后下降的趋势。这是因为随铅胁迫强度的增强,细胞中活性氧积累超过限度,使得SOD、APX和AsA分解或失活[33]。2 500 mg·kg−1铅处理下GSH质量分数下降可能因为GSH是合成螯合肽(PCs)的底物,直接与重金属离子结合[34]。
可溶性糖及脯氨酸可以有效防止细胞质渗透势过高造成的细胞脱水和膜脂过氧化,维持细胞代谢活动的正常[20]。有研究表明八仙花叶片中的可溶性糖及脯氨酸质量分数在铅处理下呈上升趋势[10],在八仙花根系中也发现了同一现象,说明可溶性糖及脯氨酸的增加是八仙花应对铅胁迫的自身调节机制。
-
本研究检测到的根系分泌物脂肪酸、醇类和有机酸在八仙花抗铅胁迫中发挥着重要作用。脂肪酸是细胞膜的重要组成成分[35],与植物对重金属胁迫的耐受性呈正相关[16]。1 500和2 500 mg·kg−1铅胁迫显著增加了脂肪酸相对含量。这与SUN等[16]研究结果相反。说明铅胁迫下八仙花通过增加脂肪酸(如棕榈酸)的相对含量,以增加膜的流动性,维持细胞膜的正常结构和功能。植物根系分泌有机酸是一种外部缓解重金属毒性的机制,有机酸能够活化重金属,影响重金属在土壤中的迁移、转化及生物有效性[14]。本研究中500 mg·kg−1铅处理下有机酸相对含量的增加提高了八仙花对铅的吸收能力和耐受能力。这是因为八仙花根系分泌的一些有机酸可以螯合土壤中的铅,增强铅的活动性,从而促进植物对铅的吸收和积累。醇类可以保持细胞的渗透平衡,抵抗胁迫引起的氧化损伤[36]。已有研究表明十八醇相对含量的增加可促进抗氧化酶活性的提升,从而增强植物对重金属的抗性[16]。本研究发现,醇类相对含量只有在1 500 mg·kg−1铅处理下显著降低,说明铅胁迫对八仙花造成了一定的氧化损伤。
根系分泌物中胺类、碳水化合物和氨基酸在应对非生物胁迫时亦发挥着重要作用[37]。胺类相对含量的增加有利于消除活性氧,保护细胞膜,是八仙花对铅胁迫的一种响应机制。铅胁迫下八仙花根系分泌物中碳水化合物比例显著下降,与杜鹃Rhododendron simsii在胁迫下碳水化合物增加不同[36]。这可能因为铅胁迫并未使八仙花将储存在体内的有机营养物通过分解代谢降解,说明八仙花对铅胁迫环境有良好的适应性。高质量分数铅胁迫增加了蛋白质降解,从而增加了氨基酸相对含量。
-
植物根系通过吸收、呼吸和分泌等一系列关键的生物功能改变土壤的生化特性,对土壤-植物系统有重要作用[24]。八仙花根系土壤pH降低是因为重金属Pb2+可以取代土壤中Ca2+、Na+或其他碱性阳离子[38]。此外,植物根系分泌的有机酸也会降低土壤pH[25]。土壤电导率与金属质量分数有关,八仙花根际土壤电导率的增加是由于Pb2+的增加,以及栽培基质湿度波动使得含盐量变高导致的[37]。铅胁迫下有机质比对照组增加。土壤有机质含丰富的官能团,拥有极强的络合能力,可与重金属结合形成难溶沉淀物质,降低重金属在土壤中的迁移性,从而有效降低植物对铅的吸收 [37−38]。
土壤酶活性与土壤养分质量分数密切相关,可指示土壤重金属污染程度[38]。随着铅质量分数的增加,八仙花根际土壤脲酶活性呈先增加后下降的趋势。原因是根系分泌物中含氮有机物(如胺类)增加,提高了土壤中氮质量分数,从而改善土壤肥力和土壤污染状况。而高质量分数铅会抑制土壤中微生物活性[39],且铅会与脲酶活性基团结合,或者与酶底物络合[38],从而抑制脲酶活性。植物根系分泌的低分子量化合物可以促进土壤微生物生长和繁殖,间接促进土壤酶活性的提高[25]。蔗糖酶和过氧化氢酶活性的提高表明了八仙花对铅污染土壤具有修复作用。
可交换离子态、碳酸盐结合态铅在土壤中易发生迁移、转化,具有较高的生物可利用度,易被植物吸收;铁锰氧化物结合态铅可以向可交换离子态、碳酸盐结合态铅转化,具有潜在的生物可利用度;有机物结合态铅可比较稳定存在于土壤中;残渣态铅能长期稳定存在于土壤中,不易于被植物吸收利用[40]。本研究中,铅胁迫下土壤有效态铅质量分数及占比的增加可能与pH降低有关。不同铅胁迫下八仙花根系分泌物组分与质量分数发生变化,使得土壤pH和氧化还原条件发生变化,土壤中高H+浓度可溶解和释放土壤中固定的铅,降低土壤赋存铅的稳定性,促进铅由难溶态向生物可利用态转化,从而有利于植物吸收。
-
铅胁迫抑制了八仙花的生长和铅的富集、转运能力,增加了细胞膜透性,对细胞膜造成氧化损伤,但八仙花对2 500 mg·kg−1铅仍具有一定耐受能力。八仙花根系通过增加抗氧化酶活性(SOD、POD)以及释放更多非酶抗氧化物质(GSH、Pro、SS),缓解铅胁迫造成的氧化损伤,还分泌更多的脂肪酸、胺类,以维持细胞结构、消除活性氧。此外铅胁迫使得土壤有机质及土壤酶活性增加,改善了土壤微生态环境。土壤pH降低、电导率值升高,提升了根际土壤有效态铅的水平,促进了八仙花对铅的吸收。本研究表明八仙花具有极强的铅耐受能力,是用于铅污染土壤修复的理想植物,未来可通过外源添加根系分泌的特定物质、改善根际特征来提高八仙花修复效率。
Effects of Pb stress on physiology and main rhizosphere characteristics of Hydrangea macrophylla
doi: 10.11833/j.issn.2095-0756.20240301
- Received Date: 2024-04-18
- Accepted Date: 2024-10-21
- Rev Recd Date: 2024-10-15
- Available Online: 2025-01-20
- Publish Date: 2025-02-20
-
Key words:
- Hydrangea macrophylla /
- Pb-contaminated soil /
- physiological response /
- Pb-chemical form /
- soil enzyme activities
Abstract:
| Citation: | SONG Yunjing, JIN Jing, ZHAO Bing. Effects of Pb stress on physiology and main rhizosphere characteristics of Hydrangea macrophylla[J]. Journal of Zhejiang A&F University, 2025, 42(1): 133−142 doi: 10.11833/j.issn.2095-0756.20240301 |











 DownLoad:
DownLoad:
