-
香榧Torreya grandis ‘Merrillii’ 隶属于红豆杉科Taxaceae榧树属Torreya常绿乔木,是中国特有的珍稀坚果植物,具有重要的经济、社会和生态价值[1]。由于香榧生物学周期长,性状易受环境等因子影响,限制了其育种工作的开展。目前,有关香榧体胚诱导、增殖和离体器官培养再生植株的研究已有一定进展,但关于利用基因工程改良香榧育种的研究尚未见报道[2]。基因工程是生物技术的核心,利用基因工程进行林木遗传改良,以提高作物产量、改善品质、增强抗性是林木分子育种的最有效途径之一[3]。1986年,研究人员通过农杆菌Agrobacterium tumefaciens介导法在杨树Populus trichocarpa×deltoides中成功开展遗传转化,开创了林木遗传转化研究的先河[4]。目前,林木基因转化方法已达10余种,其中,农杆菌介导法因具有操作简单、拷贝数低、转化效率高、重复性好、发生基因沉默率低等优点而被广泛应用[5]。随着木本植物遗传转化研究的不断发展,苹果Malus pumila等重要果树农杆菌介导的遗传转化体系也相继建立,为经济林果树种定向育种提供了高效的方法和手段。1989年,率先成功实现苹果转基因[6],包括早花、矮化、抗病等基因的转化,且有多个品种已成功获得转基因植株。1996年,梨Pyrus communis的遗传转化首见报道[7]。目前,在豆梨P. calleryanana和砂梨P. pyrifolia等均有报道。此外,樱桃Prunus cerasus、李P. salicina等其他水果也实现了遗传转化。然而,果树的遗传转化研究大多数集中于水果,对于坚果树种遗传转化的研究尚处于起步阶段[8]。坚果为包裹着坚硬外壳的植物种子统称[9]。坚果类食品富含不饱和脂肪酸,具有较好的抗氧化和抗衰老活性,对心脑血管等疾病具有良好的预防作用[10]。坚果分为树坚果和籽坚果。树坚果是指具有坚硬外壳的木本植物的籽粒,包括核桃Juglans regia、巴旦木Amygdalus communis、榛子Corylus heteropylla、山核桃Carya cathayensis、香榧等。坚果树种多数为多年生木本植物,组织细胞中含有大量的酚类化合物和单宁等物质,导致采用基因工程技术进行种质资源创新难度较大[11]。目前,树坚果中核桃的基因工程技术研究进展最为深入。自从MCGRANAHAN等[12]1988年成功开展了农杆菌介导的核桃遗传转化以来,科学家已对多个核桃树种进行了基因组测序[13-14],构建了良好的遗传转化体系[15],并对多个重要基因开展了功能验证,获得了多份创新种质资源,开创了果树基因工程的新局面[16]。香榧作为一种重要的树坚果,基因工程研究进展缓慢,遗传转化体系尚不成熟,限制了香榧种质创新和产业发展。本研究以香榧幼胚为转基因受体材料,从幼胚胚龄的选择、农杆菌侵染浓度和时间、羧苄青霉素质量浓度以及阳性筛选时潮霉素质量浓度对香榧遗传转化条件进行探索和优化,利用绿色荧光蛋白(GFP)信号检测分析转化效率,并通过聚合酶链式反应(PCR)检测进行阳性鉴定,以期初步建立农杆菌介导的香榧遗传转化体系,为香榧重要基因功能验证及种质创新提供重要技术体系。
-
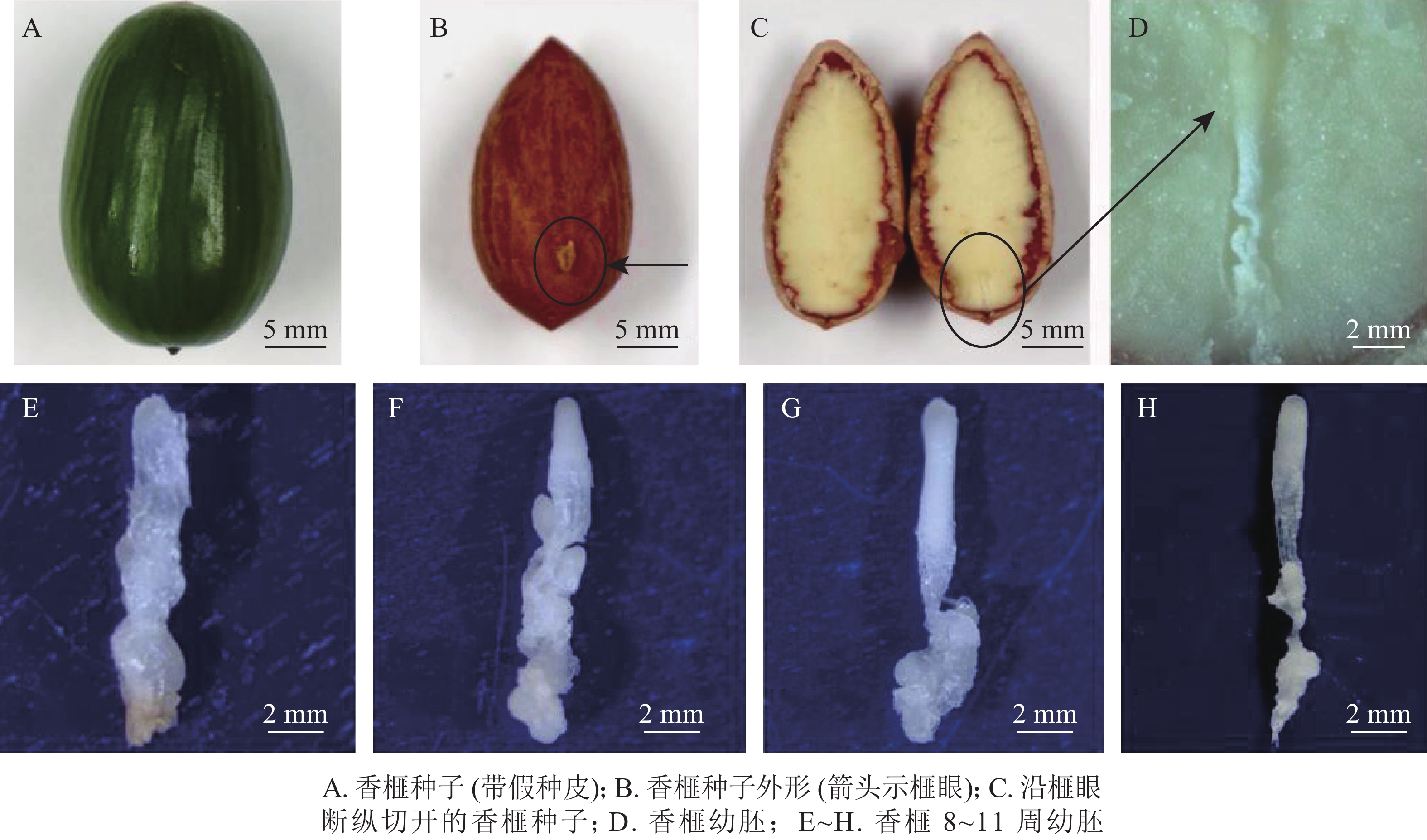
于2018年7月5—26日(种子突破种鳞后第8~11周,以下简称第8~11周)采集生长健壮的香榧种子,经消毒后,用无菌修枝剪从种子榧眼端(珠孔端)纵向剪开,剥取完整幼胚(图1A~D)待用。具体消毒步骤参照龚丽等[17]的方法。
-
采用携带GFP报告基因的pCAMBIA1300-GFP载体,内含卡那霉素抗性基因和潮霉素抗性基因[18];农杆菌菌株GV3101。
-
①农杆菌的活化与培养。将−80 ℃冰箱中保存的携带pCAMBIA1300-GFP载体的GV3101农杆菌菌株接种至含有50 mg·L−1卡那霉素的LB固体培养基中划线培养,28 ℃恒温培养箱活化培养2 d。挑取若干单菌落分别放置于盛有附加50 mg·L−1卡那霉素和50 mg·L−1利福平的LB液体培养基的无菌离心管中,28 ℃恒温振荡培养箱继续培养,至菌液混浊。②侵染液的制备。取上述适量菌液稀释10倍,分光光度计下测定吸光度[D(600)]。侵染液制备所需菌液体积按照如下公式计算:所需菌液量(mL)=[所需侵染量(mL)×所需菌液D(600)]/[实测D(600)×10]。计算得出所需菌液体积后,在超净工作台上用移液枪吸取所需菌液至无菌带盖离心管中6 000 r·min−1转速下离心10 min,弃上清液,加入2 mL含有 0.1 mg·L−1萘乙酸和40.0 mg·L−1乙酰丁香酮的1/2 SH液体共培养基,吸打混匀成悬浮液后,再加液体共培养基至所需菌液侵染量,将离心管放回至28 ℃恒温振荡培养箱继续培养1~2 h,待用。
-
①香榧幼胚最佳胚龄筛选。采用第8~11周的香榧幼胚,用农杆菌侵染[D(600)为0.5],并添加乙酰丁香酮共培养后接种至农杆菌脱菌培养基(1/2 SH+0.1 mg·L−1萘乙酸+2.0 g·L−1活性炭+30.0 g·L−1蔗糖+200.0 mg·L−1羧苄青霉素+12.0 g·L−1琼脂)培养4周,分别统计筛选过程中香榧幼胚的污染率、成活率、愈伤组织诱导率以及体细胞胚(简称体胚)发生率。每个处理重复3次,每次处理30个幼胚。②最佳农杆菌侵染吸光度筛选。将农杆菌菌液吸光度设置为4个水平[D(600)为0.3、0.5、0.8、1.0],对香榧幼胚侵染15 min,乙酰丁香酮共培养3 d后接种至脱菌培养基(同①)培养4周,统计幼胚污染率、成活率、愈伤组织诱导率和体胚发生率,确定适宜转化用的菌液浓度。每个处理重复3次,每次处理30个幼胚。③最佳农杆菌侵染时间筛选。采用 D(600)为0.5的农杆菌菌液对幼胚分别侵染5、10、15、20、30 min,共培养3 d后转接至脱菌培养基(同①)培养4周,统计污染率、成活率、愈伤组织诱导率以及体胚发生率,以确定适宜转化用的菌液浓度。每个处理重复3次,每次处理30个幼胚。④最佳羧卞青霉素质量浓度筛选。将共培养后的香榧幼胚接种在含有 0.1 mg·L−1萘乙酸和不同质量浓度羧卞青霉素(100、200、300、400 mg·L−1)的1/2 SH固体培养基中进行抗生素质量浓度筛选。每个处理重复3次,3 d继代培养1次,4周后统计污染率、成活率、愈伤组织诱导率以及体胚发生率,以确定适宜的羧苄青霉素质量浓度。
-
选取生长良好的香榧幼胚在最佳浓度农杆菌侵染液中,悬浮培养最佳侵染时间后,将幼胚转接至附加萘乙酸(0.1 mg·L−1)和乙酰丁香酮(40 mg·L−1)的1/2 SH固体培养基中共培养3 d后,再转接至脱菌培养基(1.2.2节④筛选出的最佳培养基)中培养至无菌。具体步骤参照ZHANG等[8]的方法。将脱菌后的香榧幼胚接种至附加0.1 mg·L−1萘乙酸和质量浓度分别为50、80、100、200 mg·L−1潮霉素的1/2 SH固体培养基中进行筛选培养,2周后转接至附加0.1 mg·L−1萘乙酸和100 mg·L−1潮霉素的1/2 SH固体培养基中进行筛选培养,隔2 d转接1次至完全脱菌并获得潮霉素抗性幼胚。
-
将经潮霉素筛选的抗性幼胚置于体式显微镜(SteREO Discovery V1)下进行450~490 nm蓝光激发,表面能观察到明亮绿色荧光的幼胚为GFP荧光信号阳性幼胚。选取潮霉素基因和GFP绿色荧光鉴定均为阳性的幼胚培养材料,利用TPS法提取DNA。利用GFP基因引物(GFP-F: 5′-GACGCACAATCCCACTATCCTT-3′, GFP-R: 5′-AACCGATGATACGAACGAAAGC-3′),以上述提取的DNA为模板进行GFP基因的PCR扩增[PCR程序为:94 ℃ 2 min;98 ℃ 10 s;68 ℃ 45 s (32个循环);72 ℃ 10 min;4 ℃保存],目的片段大小为750 bp。PCR程序结束后,10 g·kg−1琼脂糖凝胶电泳检测,条带符合预期大小的确定为转化成功的香榧幼胚培养物。
-
受体材料污染率、成活率及GFP阳性率等数据采用SPSS 20.0软件进行统计分析,其中包括平均值和方差分析。计算公式如下:污染率=污染幼胚数/侵染总体胚数×100%;成活率=成活幼胚数/侵染总体胚数×100%; GFP阳性率=GFP阳性幼胚数/成活幼胚数×100%。
-
本研究采用处于第8~11周的香榧幼胚为材料进行农杆菌介导的遗传转化(图1E~H)。第8~11周分别为胚胎选择初期、胚胎选择中期、胚胎选择晚期和优势胚完全发育期。结果表明(表1):4个胚龄的幼胚转化过程中污染率和成活率均存在显著差异(P<0.05)。随着香榧幼胚龄的增加,抗逆性增强,污染率降低,成活率提高。第10周和第11周成活率分别为52.1%和52.3%。香榧幼胚胚性感受态具有较大差异,第10周时愈伤组织诱导率下降为17.9%,但体胚发生率最高,达17.3%;第11周时愈伤组织诱导率和体胚发生率均下降,分别为10.7%和5.6%。尽管香榧第8~11周幼胚均可用于农杆菌介导的遗传转化,但综合污染率、成活率、愈伤组织诱导率和体胚发生率均表明采用第10周香榧幼胚进行农杆菌介导的遗传转化效果最佳。
表 1 香榧幼胚胚龄对遗传转化的影响
Table 1. Different growth stages of embryos on the transformation efficiency in T. grandis ‘Merrillii’
采样时
间/周污染率/
%成活率/
%愈伤组织
诱导率/%体胚发生
率/%8 22.3±0.3 a 35.6±1.3 b 18.1±0.6 a 0.0±0.0 c 9 21.5±0.5 a 40.9±2.3 b 20.1±1.6 a 0.0±0.0 c 10 18.8±0.3 b 52.1±2.0 a 17.9±0.3 b 17.3±1.2 a 11 18.7±0.6 b 52.3±1.3 a 10.7±0.7 b 5.6±0.5 b 说明:同列不同小写字母表示差异显著(P<0.05) -
表2表明:随着菌液D(600)的升高,污染率逐渐上升,成活率逐渐下降。过高的污染率会增加大量的前期采样、灭菌、侵染、共培养及后期脱菌培养的工作量。当菌液D(600)=0.3时,污染率最低,成活率最高,但愈伤组织诱导率和体胚发生率都比D(600)=0.5时低。随着D(600)的升高,愈伤组织诱导率和体胚发生率先上升后下降。当D(600)=0.5时,愈伤组织诱导率和体胚发生率最高,分别为17.9%和17.3%。因此,综合考虑,D(600)=0.5时是最佳侵染浓度(表2)。
表 2 农杆菌菌液浓度对香榧幼胚遗传转化的影响
Table 2. Different concentrations of bacteria on the impact of transformation in T. grandis ‘Merrillii’
D(600) 污染率/
%成活率/
%愈伤组织
诱导率/%体胚发生
率/%0.3 42.0±0.8 d 62.1±1.4 a 15.2±1.0 a 6.9±0.9 b 0.5 48.2±0.6 c 58.7±3.8 a 17.9±1.5 a 17.3±0.3 a 0.8 63.1±3.3 b 36.6±0.8 b 8.9±0.5 b 4.9±0.3 c 1.0 71.5±1.4 a 14.1±0.2 c 0.0±0.0 c 0.0±0.0 d 说明:同列不同小写字母表示差异显著(P<0.05) -
表3表明:随着侵染时间的延长,污染率逐渐升高,成活率逐渐下降,侵染5 min时,污染率最低,成活率最高;当侵染30 min时,污染率最高,达100%。不同侵染时间下,各处理间愈伤组织诱导率和体胚发生率均存在显著差异(P<0.05),侵染10 min时,愈伤组织诱导率和体胚发生率均为最高,分别达17.5%和17.1%;侵染20~30 min时,愈伤组织诱导率和体胚发生率均下降至0。综合以上指标,选择10 min为最佳侵染时间。
表 3 农杆菌菌液不同侵染时间对香榧幼胚遗传转化的影响
Table 3. Effect of different infection times on transformation in T. grandis ‘Merrillii’
侵染时间/
min污染率/
%成活率/
%愈伤组织
诱导率/%体胚发生
率/%5 35.2±0.4 e 72.1±2.3 a 16.2±0.6 b 15.3±0.3 b 10 45.1±0.7 d 65.3±0.6 b 17.5±0.3 a 17.1±1.0 a 15 49.3±1.4 c 60.5±1.4 c 10.8±0.3 c 3.5±0.4 c 20 72.5±0.5 b 42.8±1.1 d 0.0±0.0 d 0.0±0.0 d 30 100.0±3.7 a 0.0±0.0 e 0.0±0.0 d 0.0±0.0 d 说明:同列不同小写字母表示差异显著(P<0.05) -
本研究选用的农杆菌种类为碱型GV3101菌株,因此,在前期研究的基础上,选用羧苄青霉素为农杆菌脱菌抗生素。表4表明:香榧幼胚成活率随着羧苄青霉素质量浓度的升高先上升后下降,当羧苄青霉素质量浓度为300 mg·L−1时成活率最高,为60.5%。随着羧苄青霉素质量浓度的升高,愈伤组织诱导率和体胚发生率均呈先上升后下降的趋势,当质量浓度为300 mg·L−1时,愈伤组织诱导率和体胚发生率最高,分别为15.8%和17.5%。因此,最佳羧苄青霉素质量浓度为300 mg·L−1。
表 4 羧苄青霉素质量浓度对香榧幼胚农杆菌脱除的影响
Table 4. Effects of carboxypenicillin concentration on the removal of Agrobacterium tumebii from T. grandis ‘Merrillii’ embryos
羧苄青霉素/
(mg·L−1)污染率/
%成活率/
%愈伤组织
诱导率/%体胚发生
率/%100 82.2±1.0 a 22.1±0.8 c 6.7±0.2 c 00.0±0.0 c 200 64.9±1.9 b 45.3±2.3 b 7.3±0.1 b 7.1±0.4 b 300 17.3±2.0 c 60.5±1.4 a 15.8±0.3 a 17.5±1.5 a 400 12.1±0.3 d 42.8±1.2 b 0.0±0.0 d 0.0±0.0 c 500 0.0±0.0 e 10.1±0.4 d 0.0±0.0 d 0.0±0.0 c 说明:同列不同小写字母表示差异显著(P<0.05) -
表5表明:随着潮霉素质量浓度的升高,幼胚成活率逐渐下降,当潮霉素质量浓度为50 mg·L−1时,成活率最高,为62.1%;当潮霉素质量浓度升高至100 mg·L−1时,成活率下降至30.5%,但当潮霉素质量浓度继续升高至200 mg·L−1,香榧幼胚培养物的成活率基本不变。当潮霉素质量浓度为50~100 mg·L−1时,各处理间愈伤组织诱导率和体胚发生率无显著性差异;当潮霉素质量浓度升高至200 mg·L-1时,愈伤组织诱导率和体胚发生率均显著下降(P<0.05)。因此, 100 mg·L−1为筛选抗性幼胚培养物的潮霉素最佳质量浓度。
表 5 潮霉素质量浓度对香榧幼胚培养物潮霉素阳性筛选的影响
Table 5. Effect of hygromycin concentration on positive screening of T. grandis ‘Merrillii’ embryo culture
潮霉素/
(mg·L−1)成活率/
%愈伤组织
诱导率/%体胚发生
率/%50 62.1±0.7 a 14.7±0.5 a 12.8±0.3 a 80 45.3±1.7 b 14.3±0.7 a 13.1±0.1 a 100 30.5±1.2 c 15.2±1.6 a 12.5±0.5 a 200 30.8±0.9 c 8.2±0.3 b 7.6±0.2 b 说明:同列不同小写字母表示差异显著(P<0.05) -
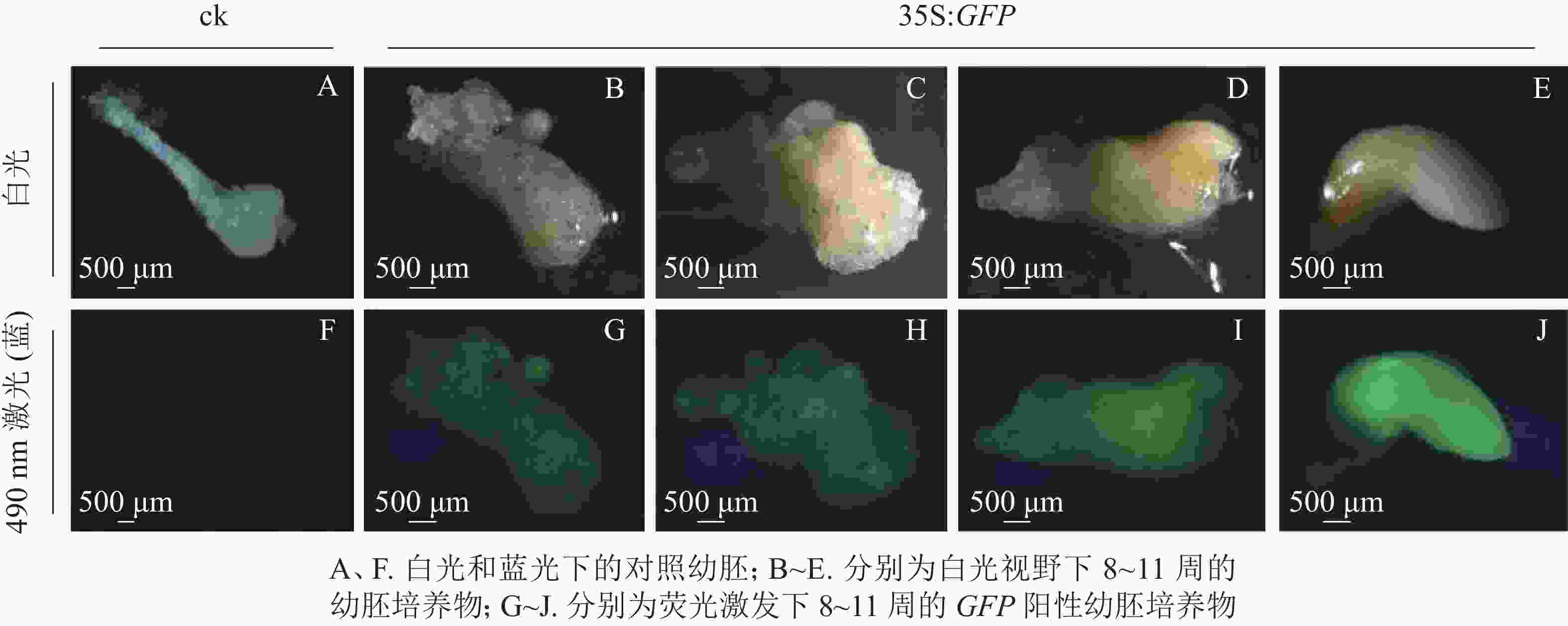
将通过筛选的潮霉素抗性幼胚置于体式显微镜下进行蓝光激发(490 nm)。结果发现:潮霉素抗性幼胚培养物中,白光视野下,胚胎选择期(第8~10周)幼胚培养物呈现乳白色至淡黄色,蓝光视野下呈现明亮的绿色荧光,各胚龄间GFP荧光阳性率无显著性差异,为75.5%~78.3%;优势胚完全发育期(第11周)GFP荧光阳性率较低,为53.0%,且幼胚不同部位的荧光表达量不一致,其中相对成熟的部位荧光表达量较低,生长旺盛单位荧光表达量较高,呈现明亮的绿色;对照幼胚培养物在白光下呈现乳白色至淡黄色,蓝光视野中无荧光激发,呈现黑色(图2)。
-
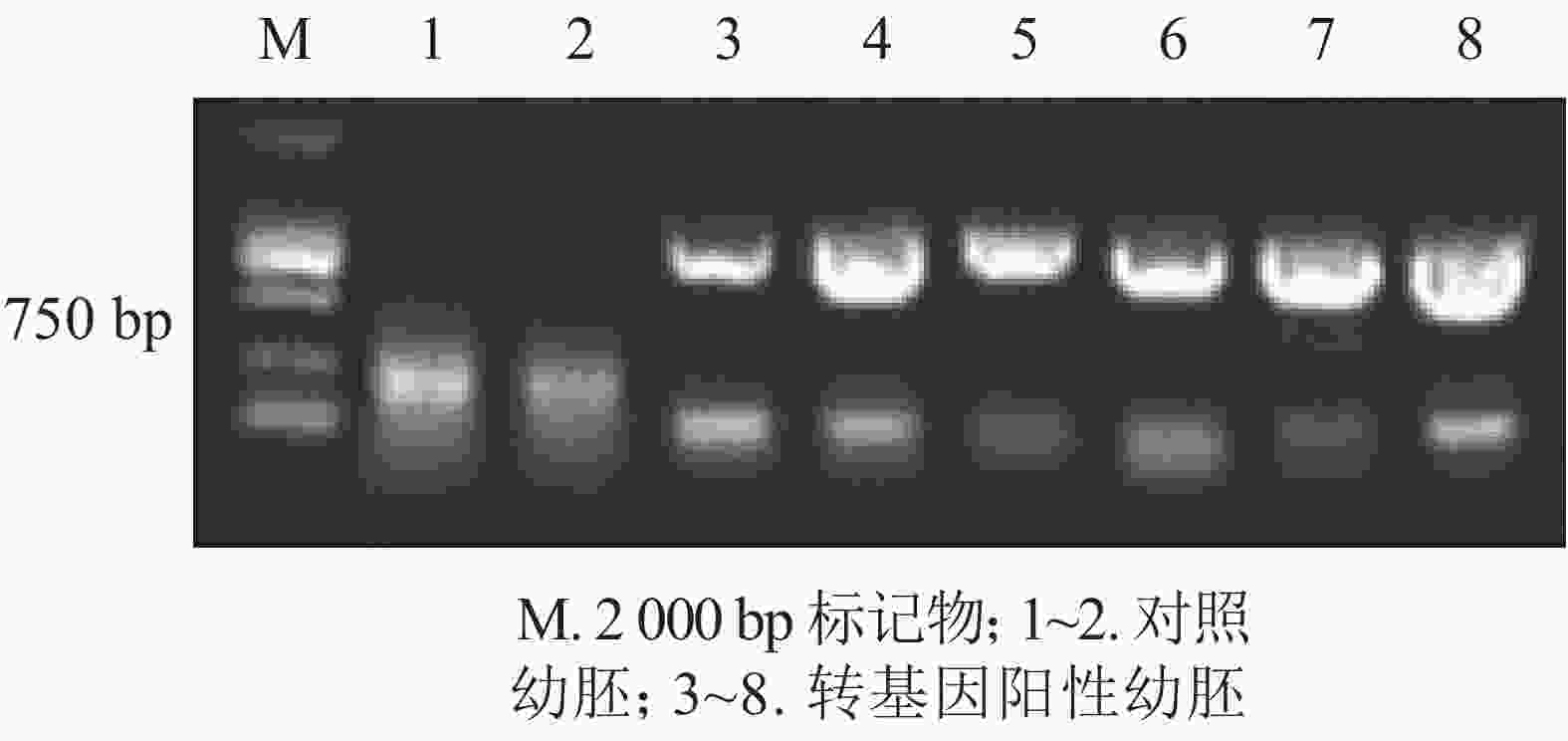
为进一步排除GFP绿色荧光假阳性,本研究选取具有潮霉素抗性且GFP强荧光信号表达的香榧幼胚培养物提取DNA并进行PCR扩增,并以同时接种未转化的幼胚为对照。香榧GFP荧光阳性幼胚培养物提取DNA进行PCR检测结果表明(图3):75%的携带潮霉素抗性且GFP荧光阳性香榧幼胚培养物可扩增出目的条带,长度约750 bp,符合预期大小(泳道3~8),而对照幼胚培养物相应无条带(泳道1~2),表明GFP基因已成功转入香榧幼胚。
-
在木本植物遗传转化中,常用的受体材料一般有花序、叶片、体细胞胚、胚性愈伤组织、合子胚、下胚轴、原生质体、茎尖及花粉等[19]。目前,林木遗传转化研究多以胚性愈伤组织、合子胚和体细胞胚为受体材料[20]。
有研究表明:同一外植体的不同部位对农杆菌转化效率也不同[21],胡杨Populus euphratica在遗传转化时使用叶片的转化率高达80%,而茎段转化率只有20%[22]。受体材料的生长发育阶段对遗传转化效率具有重要影响[23]。处于旺盛分裂期的林木细胞或组织是农杆菌转化成功的基础。一般来说,发育早期的组织细胞分裂能力较强[24]。方宏筠等[25]在研究黑核桃Juglans nigra体胚遗传转化时发现:球形胚、鱼雷胚等有较高的转化效率,而发育晚期的子叶胚很难诱导出抗性体细胞胚状体,这可能与其分裂能力低有关。DAI等[26]选用葡萄Vitis vinifera胚胎发育过程中的3个阶段(胚性愈伤组织、原胚团、体胚)为遗传转化的受体材料,结果显示:仅原胚团样品得到了32个转基因株系。选择幼胚作为外植体,易产生胚性愈伤组织,且分化和再生能力较强,缩短了再生所需时间,提高了遗传转化效率[27]。香榧胚性愈伤组织结构特殊,胚性细胞团结构疏松脆弱,在农杆菌侵染过程中极易受到不可逆的损害,大大影响了后续发育的活力。因此,本研究以种子突破种鳞后第8~11周的香榧幼胚为农杆菌介导的遗传转化受体材料,发现处于胚胎选择期的香榧幼胚遗传转化能力较强,GFP荧光阳性率高于优势胚完全发育期,与该时期幼胚具有较强的胚性感受态有关[17]。
-
菌液侵染是农杆菌介导的遗传转化的第1步,涉及诸多影响因子,包括农杆菌菌液浓度、侵染时间等。一般来讲,菌液浓度过高,脱菌时抗生素不能抑制农杆菌生长,而且易使外植体产生过敏反应;当菌液浓度较低时,外植体表面的菌液不足以侵染植物组织细胞[28]。刘晓晨[15]在研究核桃体胚转化条件时发现:在侵染时间相同情况下,阳性率随着侵染液浓度提高呈上升趋势,但污染率也随之提高;仝铸等[29]在建立柠檬Citrus limon遗传转化体系时发现:菌液D(600)为0.6时胚轴再生率达到最高,D(600)高于0.6胚轴再生率随之下降。因此,选择合适的菌液吸光度是高效转化体系的关键。本研究中,农杆菌D(600)为0.5时香榧幼胚转化效率最高。
农杆菌侵染时间的长短也因植物材料不同而异,适宜的时间可以确保农杆菌对植物细胞充分稳定的吸附,时间过短不能完成农杆菌有效侵染,导致转化效率过低甚至转化失败,而过长的侵染时间容易导致材料过度污染甚至死亡。杜丽等[30]研究樟树Cinnamomum camphora胚性愈伤组织遗传转化时发现:转化效率在侵染时间超过40 min后呈下降趋势。糜瑶琦[31]在研究美国山核桃Carya illinoinensis遗传转化条件时发现:侵染15 min时β-葡萄糖苷酸酶(GUS)表达率最高,达61.52%,超过15 min后呈下降趋势。在本研究的香榧幼胚转化中,最佳侵染时间为10 min,且不同农杆菌菌液吸光度和侵染时间处理下,污染率、成活率、愈伤组织诱导率以及体胚发生率均具显著性差异。
农杆菌介导遗传转化的另一个重要环节为脱菌培养。筛选出适合的抑菌抗生素对于农杆菌介导的遗传转化至关重要[32]。林木遗传转化中常用的抑菌剂有头孢霉素和羧苄青霉素等[33]。黄建[34]研究枣Ziziphus jujuba遗传转化时发现,低浓度的羧苄青霉素促进叶片外植体形成愈伤组织,而浓度过高有抑制作用。MONDAL等[35]在研究茶Camellia sinensis遗传转化中发现:头孢霉素和羧苄青霉素协同使用,可有效抑制农杆菌的活性。坚果树种对农杆菌脱菌抗生素非常敏感,在加有抗生素压力的抑菌培养基上生长能力较差。优化核桃体胚遗传转化条件时,使用250 mg·L−1羧苄青霉素时体胚转化率最高,达28.0%,随后体胚转化率逐渐下降[36]。在本次香榧幼胚的转化中,300 mg·L−1羧苄青霉素为最佳脱菌质量浓度。
目前,坚果类树种的遗传转化仍存在较多问题需要进一步探究和解决。坚果树种转化方法单一是限制其遗传转化体系建立的一个重要因素[19]。此外,坚果类树种体胚再生频率低、重复性差的自身特性是影响转化效率的主要原因,甚至有些坚果树种难以建立再生体系,致使转化无法进行。这一特性是导致坚果类树种遗传转化研究和应用滞后的主要原因[37]。本研究在受体材料选择、农杆菌侵染时间、侵染浓度、抗生素质量浓度等方面进行了初步研究,成功获得了香榧转化幼胚培养物。研究结果可为香榧重要基因遗传转化和功能验证打下良好基础,同时也为香榧种质创新提供重要技术平台。
Agrobacterium tumefaciens-mediated genetic transformation system of Torreya grandis ‘Merrillii’ immature embryos
-
摘要:
目的 以香榧Torreya grandis ‘Merrillii’幼胚为受体材料,开展农杆菌Agrobacterium tumefaciens介导的遗传转化研究,揭示影响香榧遗传转化的关键因素,以建立农杆菌介导的香榧幼胚遗传转化体系。 方法 以香榧种子突破种鳞后第8周至第11周的幼胚作为转基因受体材料,比较受体胚龄、农杆菌菌液浓度、侵染时间、抗生素质量浓度对遗传转化效率的影响,并采用潮霉素、绿色荧光蛋白GFP表达及GFP基因聚合酶链式反应对遗传转化香榧幼胚培养物进行阳性筛选。 结果 随着胚龄的增加,香榧幼胚抗逆性增强,污染率降低,成活率提高,第10周和第11周幼胚成活率分别为52.1%和52.3%。农杆菌菌液浓度和侵染时间均对香榧幼胚的污染率、成活率、愈伤组织诱导率以及体胚发生率具有显著影响(P<0.05)。菌液吸光度[D(600)]为0.5时,香榧幼胚愈伤组织诱导率和体胚发生率最高,分别为17.9%和17.3%;侵染时间为10 min时,愈伤组织诱导率和体胚发生率为最高,分别达17.5%和17.1%。羧苄青霉素对于受体材料的农杆菌脱除具有显著影响(P<0.05),当羧苄青霉素质量浓度为300 mg·L−1时,成活率、愈伤组织诱导率和体胚发生率均最高,分别为60.5%、15.8%和17.5%。潮霉素对于香榧幼胚培养物的阳性筛选具有良好的效果,当潮霉素质量浓度为100 mg·L−1时,成活率为30.5%。 结论 不同胚龄香榧幼胚遗传转化效果不同,突破种鳞后第10周香榧幼胚遗传转化效果良好;当农杆菌菌液D(600)为0.5,侵染时间为10 min时,转化效果最佳;300 mg·L−1为羧苄青霉素脱除农杆菌的最佳质量浓度;100 mg·L−1是潮霉素阳性香榧幼胚筛选的最佳质量浓度。图3表5参37 -
关键词:
- 香榧 /
- 幼胚 /
- 农杆菌介导的遗传转化 /
- 羧苄青霉素 /
- 潮霉素
Abstract:Objective The purpose is to explore genetic transformation mediated by Agrobacterium tumefaciens using Torreya grandis ‘Merrillii’ immature embryos as receptors, so as to reveal key factors affecting its genetic transformation and establish A. tumefaciens mediated transformation system of T. grandis ‘Merrillii’ embryos. Method The immature embryos of T. grandis ‘Merrillii’ seeds of 8 to 11 weeks after breaking through seed scales were used as transgenic acceptors. The effects of embryo age, A. tumefaciens concentration, infection time and antibiotic concentration on genetic transformation efficiency were compared. Hygromycin, GFP fluorescent expression and GFP gene polymerase chain reaction were used for positive screening and selection of immature embryo culture. Result The stress resistance increased, the contamination rate decreased and the survival rate increased with the increase of embryo age. The survival rates of 10-week and 11-week young embryos were 52.1% and 52.3%, respectively. The contamination rate, survival rate, callus induction rate and somatic embryogenesis rate of young embryos were significantly affected by the concentration of A. tumefaciens and infection time (P<0.05). When the liquid optical density [D(600)] was 0.5, the callus induction rate and somatic embryogenesis rate of young embryos were the highest, which were 17.9% and 17.3% respectively. When the infection time was 10 minutes, the callus induction rate and somatic embryogenesis rate were the highest, reaching 17.5% and 17.1% respectively. Carbenicillin had a significant effect on the removal of A. tumefaciens (P<0.05). When the concentration was 300 mg·L−1, the survival rate, callus induction rate and somatic embryogenesis rate were the highest, which were 60.5%, 15.8% and 17.5% respectively. The concentration of hygromycin in different treatments had a good effect on the positive screening of young embryo culture. The survival rate was 30.5% when the concentration of hygromycin was 100 mg·L−1. Conlusion Immature embryos at the 10th week after the breakthrough of scale, 0.5 [D(600)], 10 minutes duration, 300 mg·L−1 carbenicillin, and 100 mg·L−1 hygromycin were the optimum conditions for genetic transformation of T. grandis ‘Merrillii’. [Ch, 3 fig. 5 tab. 37 ref.] -
表 1 香榧幼胚胚龄对遗传转化的影响
Table 1. Different growth stages of embryos on the transformation efficiency in T. grandis ‘Merrillii’
采样时
间/周污染率/
%成活率/
%愈伤组织
诱导率/%体胚发生
率/%8 22.3±0.3 a 35.6±1.3 b 18.1±0.6 a 0.0±0.0 c 9 21.5±0.5 a 40.9±2.3 b 20.1±1.6 a 0.0±0.0 c 10 18.8±0.3 b 52.1±2.0 a 17.9±0.3 b 17.3±1.2 a 11 18.7±0.6 b 52.3±1.3 a 10.7±0.7 b 5.6±0.5 b 说明:同列不同小写字母表示差异显著(P<0.05) 表 2 农杆菌菌液浓度对香榧幼胚遗传转化的影响
Table 2. Different concentrations of bacteria on the impact of transformation in T. grandis ‘Merrillii’
D(600) 污染率/
%成活率/
%愈伤组织
诱导率/%体胚发生
率/%0.3 42.0±0.8 d 62.1±1.4 a 15.2±1.0 a 6.9±0.9 b 0.5 48.2±0.6 c 58.7±3.8 a 17.9±1.5 a 17.3±0.3 a 0.8 63.1±3.3 b 36.6±0.8 b 8.9±0.5 b 4.9±0.3 c 1.0 71.5±1.4 a 14.1±0.2 c 0.0±0.0 c 0.0±0.0 d 说明:同列不同小写字母表示差异显著(P<0.05) 表 3 农杆菌菌液不同侵染时间对香榧幼胚遗传转化的影响
Table 3. Effect of different infection times on transformation in T. grandis ‘Merrillii’
侵染时间/
min污染率/
%成活率/
%愈伤组织
诱导率/%体胚发生
率/%5 35.2±0.4 e 72.1±2.3 a 16.2±0.6 b 15.3±0.3 b 10 45.1±0.7 d 65.3±0.6 b 17.5±0.3 a 17.1±1.0 a 15 49.3±1.4 c 60.5±1.4 c 10.8±0.3 c 3.5±0.4 c 20 72.5±0.5 b 42.8±1.1 d 0.0±0.0 d 0.0±0.0 d 30 100.0±3.7 a 0.0±0.0 e 0.0±0.0 d 0.0±0.0 d 说明:同列不同小写字母表示差异显著(P<0.05) 表 4 羧苄青霉素质量浓度对香榧幼胚农杆菌脱除的影响
Table 4. Effects of carboxypenicillin concentration on the removal of Agrobacterium tumebii from T. grandis ‘Merrillii’ embryos
羧苄青霉素/
(mg·L−1)污染率/
%成活率/
%愈伤组织
诱导率/%体胚发生
率/%100 82.2±1.0 a 22.1±0.8 c 6.7±0.2 c 00.0±0.0 c 200 64.9±1.9 b 45.3±2.3 b 7.3±0.1 b 7.1±0.4 b 300 17.3±2.0 c 60.5±1.4 a 15.8±0.3 a 17.5±1.5 a 400 12.1±0.3 d 42.8±1.2 b 0.0±0.0 d 0.0±0.0 c 500 0.0±0.0 e 10.1±0.4 d 0.0±0.0 d 0.0±0.0 c 说明:同列不同小写字母表示差异显著(P<0.05) 表 5 潮霉素质量浓度对香榧幼胚培养物潮霉素阳性筛选的影响
Table 5. Effect of hygromycin concentration on positive screening of T. grandis ‘Merrillii’ embryo culture
潮霉素/
(mg·L−1)成活率/
%愈伤组织
诱导率/%体胚发生
率/%50 62.1±0.7 a 14.7±0.5 a 12.8±0.3 a 80 45.3±1.7 b 14.3±0.7 a 13.1±0.1 a 100 30.5±1.2 c 15.2±1.6 a 12.5±0.5 a 200 30.8±0.9 c 8.2±0.3 b 7.6±0.2 b 说明:同列不同小写字母表示差异显著(P<0.05) -
[1] 黎章矩, 戴文圣. 中国香榧[M]. 北京: 科学出版社, 2007. LI Zhangju, DAI Wensheng. Torreya grandis ‘Merrillii’ of China[M]. Beijing: Science Press, 2007. [2] JIAN Shugang, SHI Suhua, ZHONG Yang, et al. Genetic diversity among south China Heritiera littoralis detected by inter-simple sequence repeats (ISSR) analysis [J]. J Genet Mol Biol, 2002, 13(4): 272 − 276. [3] ROOKE L, LINDSEY K. Potato transformation [J]. Method Mol Biol, 1998, 81(4): 353 − 358. [4] PARONS T J, SINKAR V P, STETTLER F, et al. Transformation of poplar by Agrobacterium tumefaciens [J]. Bio/Tech-nology, 1986, 4: 533 − 536. [5] HIGGINES E S, HULME J S, SHIELDS R. Early events in transformation of potato by Agrobacterium tumefaciens [J]. Plant Sci, 1992, 82(1): 109 − 118. [6] JAMES D J, PASSEY A J, BARBARA D J, et al. Genetic transformation of apple (Malus pumila Mill) using a disarmed ti-binary vector [J]. Plant Cell Rep, 1989, 7(8): 658 − 661. [7] MOURGUES F, CHEVREAU E, LAMBERT C, et al. EfficientAgrobacterium-mediated transformation and recovery of transgenic plants from pear (Pyrus comnunis L.) [J]. Plant Cell Rep, 1996, 16(3/4): 245 − 249. [8] ZHANG Qixiang, WALAWAGE S L, TRICOLI D M, et al. A red fluorescent protein (DsRED) from Discosoma sp. as a reporter for gene expression in walnut somatic embryos [J]. Plant Cell Rep, 2015, 34: 861 − 869. [9] 郗荣庭, 张毅萍. 中国核桃[M]. 北京: 中国林业出版社, 1992: 1 − 5. XI Rongting, ZHANG Yiping. China Walnut [M]. Beijing: China Forestry Press, 1992: 1 − 5. [10] MORGILLO S, HILL A M, COATES A M. The effects of nut consumption on vascular function [J/OL]. Nutrients, 2019, 11(1): 116[2021-03-25]. doi: 10.3390/nu11010116. [11] SAVAGE G P. Chemical composition of walnuts (Juglans regia L.) grown in New Zealand [J]. Plant Food Hum Nutr, 2001, 56(1): 75 − 82. [12] MCGRANHAN G H, LESLIE C A, URATSU S L, et al. Agrobacterium-mediated transformation of walnut somatic embryos and regeneration of transgenic plants [J]. Bio/Technology, 1988, 6: 800 − 804. [13] MARTINEZ P J, CREPEAU M W, PUIU D, et al. The walnut (Juglans regia) genome sequence reveals diversity in genes coding for the biosynthesis of non-structural polyphenols [J]. Plant J, 2016, 87: 507 − 532. [14] ZHANG Junpei, ZHANG Wenting, JI Feiyang, et al. A high-quality walnut genome assembly reveals extensive gene expression divergences after whole-genome duplication [J]. Plant Biotechnol J, 2020, 18(9): 1848 − 1850. [15] 刘晓晨. 核桃遗传转化体系及嫁接砧穗间基因转导探究[D]. 杭州: 浙江农林大学, 2017. LIU Xiaochen. The Establishment of Genetic Transformation in Walnut (Juglans regia L.) and the Study on Gene Transduction between Grafts[D]. Hangzhou: Zhejiang A&F University, 2017. [16] ESCOBAR M A, JAEIN P, POLITO V S, et al. Using GFP as a scorable marker in walnut somatic embryo transformation [J]. Ann Bot, 2000, 85(6): 831 − 835. [17] 龚丽, 胡恒康, 胡渊渊, 等. 香榧幼胚发育与胚性感受态之间的相关性[J]. 浙江农林大学学报, 2018, 35(5): 861 − 867. GONG Li, HU Hengkang, HU Yuanyuan, et al. Immature embryo development and embryogenic frequency in Torreya grandis ‘Merrillii’ [J]. J Zhejiang A&F Univ, 2018, 35(5): 861 − 867. [18] 张佳琦, 胡恒康, 徐川梅, 等. 核桃JrGA2ox基因的克隆、亚细胞定位及功能验证[J]. 林业科学, 2019, 55(2): 50 − 60. ZHANG Jiaqi, HU Hengkang, XU Chuanmei, et al. Cloning, subcellular localization and dunction verification of gibberellin 2-oxidase gene in walnut (Juglans regia) [J]. Sci Silv Sin, 2019, 55(2): 50 − 60. [19] 王瑶, 林木兰, 沈锡辉, 等. 农杆菌介导的木本植物遗传转化[J]. 生物技术通报, 1999, 15(6): 23 − 27. WANG Yao, LIN Mulan, SHEN Xihui, et al. Xylophyta genetic transformation byAgrobacterium [J]. Biotechnol Inf, 1999, 15(6): 23 − 27. [20] 宋跃, 甄成, 张含国, 等. 长白落叶松胚性愈伤组织诱导及体细胞胚胎发生[J]. 林业科学, 2016, 52(10): 45 − 54. SONG Yue, ZHEN Cheng, ZHANG Hanguo, et al. Embryogenic callus induction and somatic embryogenesis from immature zygotic embryos of Larix olgensis [J]. Sci Silv Sin, 2016, 52(10): 45 − 54. [21] CONFALONIERI M, BALESTRAZZI A, BISOFFI S. Genetic transformation ofPopulus nigra by Agrobacterium tumefaciens [J]. Plant Cell Rep, 1994, 13(5): 256 − 261. [22] 范源伟, 刘挨枝, 王华芳. 胡杨转基因体系的建立[J]. 植物学报, 2009, 44(6): 728 − 734. FAN Yuanwei, LIU Aizhi, WANG Huafang. Transformation of Populus euphratica [J]. Chin Bull Bot, 2009, 44(6): 728 − 734. [23] MARITINEZ-TRUJILLO M M, CABRERA-PONCE J L, HERRERA-ESTRELLA L. Improvement of rice transformation using bombardment of scutellum-derived calli [J]. Plant Mol Biol Rep, 2003, 21(4): 429 − 437. [24] MCGRANAHAN G H, LESLIE C A, DANDEREKAR A M. Transformation of pecan and regeneration of transgenic plants [J]. Plant Cell Rep, 1993, 12(11): 634 − 638. [25] 方宏筠, 王关林. 黑核桃体细胞胚状体发生及其基因转化系统的建立[J]. 园艺学报, 2000, 27(6): 406 − 411. FANG Hongyun, WANG Guanlin. Somatic embryogenesis of Juglans nigria L. and establishment of gene transformation system of walnut [J]. Acta Hortic Sin, 2000, 27(6): 406 − 411. [26] DAI Lingmin, ZHOU Qi, LI Ruimin, et al. Establishment of a picloram-induced somatic embryogenesis system inVitis vinifera cv. chardonnay and genetic transformation of a stilbene synthase gene from wild-growing Vitis species [J]. Plant Cell Tissue Organ Cult, 2015, 121(2): 397 − 412. [27] 王世玉. 高频再生的玉米成熟胚遗传转化受体系统的建立[D]. 武汉: 华中农业大学, 2007. WANG Shiyu. Establishment of High-frequency Regeneration Ability Transgenic Acceptor System from Mature Embryos of Maize (Zea Mays L.)[D]. Wuhan: Central China Agricultural University, 2007. [28] 张福丽, 陈龙, 李成伟. 农杆菌介导的植物转基因影响因素[J]. 生物技术通报, 2012(7): 14 − 19. ZHANG Fuli, CHEN Long, LI Chengwei. Factors influencing Agrobacterium-mediated plant genetic transformation [J]. Biotechnol Bull, 2012(7): 14 − 19. [29] 仝铸, 何利刚, 吴黎明, 等. 柠檬遗传转化体系的建立及优化[J]. 湖北农业科学, 2014, 53(9): 2100 − 2102, 2106. TONG Zhu, HE Ligang, WU Liming, et al. Establishment and optimization of genetic transformation system of lemon[ Citrus limon (L.) Burm. f. ] [J]. Hubei Agric Sci, 2014, 53(9): 2100 − 2102, 2106. [30] 杜丽, 庞振凌, 周索, 等. 香樟胚性愈伤组织遗传转化体系建立[J]. 林业科学, 2008, 44(4): 54 − 59. DU Li, PANG Zhenling, ZHOU Suo, et al. Establishment of Agrobacterium-mediated transformation system of embryogenic calli ofCinnamomum camphora [J]. Sci Silv Sin, 2008, 44(4): 54 − 59. [31] 糜瑶琦. 薄壳山核桃遗传转化体系的构建[D]. 杭州: 浙江农林大学, 2017. MI Yaoqi. Construction of Pecan Genetic Transformation System[D]. Hangzhou: Zhejiang A&F University, 2017. [32] 何光源. 植物基因工程[M]. 北京: 清华大学出版社, 2007. HE Guangyuan. Plant Gene Engineering[M]. Beijing: Tsinghua University Press, 2007. [33] LIN J J, ASSAD G N, KUO J. Plant hormone effect of antibiotics on the transformation efficiency of plant tissues byAgrobacterium tumefaciens cells [J]. Plant Sci, 1995, 109(2): 171 − 177. [34] 黄建. 农杆菌介导S6PDH基因转化枣树的研究[D]. 杨凌: 西北农林科技大学, 2006. HUANG Jian. Study on Agrobacterium-mediated Transformation of Zyziphus jujube with S6PDH Gene[D]. Yangling: Northwest A&F University, 2006. [35] MONDAL T, BHATTACHARYA A, AHUJA P S, et al. Transgenic tea [Camellia sinensis (L.) O. Kuntze cv. Kangra Jat] plants obtained byAgrobacterium-medliated transformation of somatic embryos [J]. Plant Cell Rep, 2001, 20(8): 712 − 720. [36] 王庆鹏. 农杆菌介导的核桃体细胞胚转基因研究[D]. 泰安: 山东农业大学, 2020. WANG Qingpeng. Agrobacterium-mediated Genetic Transformation of Walnut (Juglans regia L.)[D]. Tai’an: Shandong Agricultural University, 2020. [37] 张勇, 张守攻, 齐力旺, 等. 杨树——林木基因组学研究的模式物种[J]. 植物学通报, 2006, 23(3): 286 − 293. ZHANG Yong, ZHANG Shougong, QI Liwang, et al. Poplar as a model for forest tree in genome research [J]. Chin Bull Bot, 2006, 23(3): 286 − 293. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20210196






 下载:
下载: