-
铅(Pb)是常见的土壤重金属污染源之一,具有不可降解性、生物累积性和高毒性,易进入食物链,最终损害人类健康[1]。植物修复是经济、环保的修复铅污染土壤的方法[2],其中,观赏植物比食用植物更具优势[3−4]。与草本观赏植物相比,木本观赏植物由于其高生物量以及发达的根系在植物修复中更具潜力[5]。八仙花Hydrangea macrophylla是常见的木本观赏植物,世界各地均有栽培。八仙花因其生长迅速、适应性强、观赏价值高等优点,广泛应用于城市园林造景、盆花栽培及鲜切花生产[6]。此外,八仙花对铅有极强的耐性,是极具修复潜力的植物[7−8]。
目前,已有的相关研究多从生长生理、重金属积累特性、组学分析角度探讨八仙花对铅的耐受差异及机制[7−12],但针对八仙花根际环境特征对其耐铅机制的影响研究未见报道。根际包含大量复杂生物及生态过程,是生态系统中最活跃的交互界面之一[13] 。重金属胁迫不仅直接影响根系生长,还会诱导植物根系分泌各种化合物,这些根系分泌物会改变土壤理化性质,影响土壤酶活性,改变根际铅的化学形态,进而影响植物对重金属的耐性及吸收[14−15] 。然而,重金属胁迫下植物根系氧化应激、根系分泌物和主要根际特征的综合响应机制鲜有研究。
本研究以八仙花为材料,研究其在不同质量分数铅处理下的铅吸收特性、根系氧化应激、根系分泌物及主要根际特征等,探讨其对重金属铅的吸收和耐受机制,为提高八仙花对铅污染土壤的修复效率和园林植物修复重金属污染土壤的应用提供参考。
-
供试土壤采自陕西省杨凌区农田0~20 cm表层土,其理化性质为:pH 7.44、有机质14.76 g·kg−1、全氮112 mg·kg−1、有效磷7.80 mg·kg−1、速效钾219 mg·kg−1、铅26.50 mg·kg−1。在风干过筛后的土壤中加入Pb(NO3)2溶液,老化4个月后得到铅质量分数为500、1 500、2 500 mg·kg−1的污染土壤。土壤实际铅质量分数分别为529.10、1 532.27、2 524.98 mg·kg−1。土壤铅梯度设置参考GB 36600—2018《土壤环境质量 建设用地土壤污染风险管控标准》及预试验结果。
选取健康、长势一致的1年生八仙花扦插苗(株高16~18 cm)栽植于塑料盆(18 cm×16 cm×11 cm)中,每盆2 kg土,处理组土壤铅为500、1 500、2 500 mg·kg−1,对照为不添加铅的洁净土壤,每盆1株,重复3次。试验于西北农林科技大学科研温室中进行,光照为自然光,室温保持在 20~25 ℃,空气相对湿度为65%。试验期间定期浇水,且未施用植保产品。50 d后收获分析。
-
试验结束后拍照记录植物样本。采用根系扫描仪系统(LA-S,万深)测定根系形态参数。将洗净的植物组织置于烘箱中,105 ℃下杀青30 min,70 ℃烘干至恒量,记录干质量。土壤样本经自然风干、粉碎、过2 mm筛后,用微波消解仪(MA165-001, Milestone)进行消化[16],采用火焰原子吸收光谱仪(PinAAcle 900F, PerkinElmer)测定铅。利用植物标准品GBW-07603(GSV-2)和土壤标准品GBW-07405(GSS-5)进行质量控制,回收率分别为102.3%和102.1%。生物富集系数(BCF)=植物铅质量分数/土壤剩余铅质量分数;转运系数(TF)=植物地上部铅质量分数/植物地下部铅质量分数[17],耐受指数=铅胁迫组根长/对照组根长×100%[18]。耐受指数可用于判定植物对重金属的耐受程度,高于50%时说明植物对重金属有较强的耐受性。
-
相对电导率(EC)和过氧化氢(H2O2)依据参考文献[19−20]的方法测定。超氧化物歧化酶(SOD)、过氧化物酶(POD)和抗坏血酸过氧化物酶(APX)活性参照参考文献[17, 21]的方法测定。抗坏血酸(AsA)和谷胱甘肽(GSH)参照文献[22]的方法测定。可溶性糖(SS)和脯氨酸(Pro)依照文献[20]的方法测定。
-
将植物根系洗净擦干后放于盛有250 mL超纯水的烧杯中,杯身用锡箔纸覆盖以避光,室温下收集6 h,过滤后得到根系分泌物[23]。根系分泌物提取与测定参照JIN等[12] 的方法。通过GC-MS分析根系分泌物的组成。采用Rtx-5MS毛细管柱(30 m × 0.25 mm内径, 0.25 μm膜厚)分离根系分泌物。自动进样器在30∶1模式下,进样1 μL衍生化样品。进样口温度为280 ℃,离子源温度为230 ℃,传输线温度为320 ℃。升温程序从50 ℃开始,保持0.5 min,然后以15 ℃·min−1升至320 ℃,保持9 min。全扫描质谱范围为35~650 m·z−1。采用NIST17数据库对代谢产物进行鉴定。
-
土壤pH值由pH计测定,土壤EC值由电导率仪测定,用重铬酸钾容量法测定土壤有机质[24] 。过氧化氢酶活性、脲酶活性和蔗糖酶活性测定参考文献[25]的方法。根际土壤不同铅形态采用Tessier连续提取法[18] 提取,再用火焰原子吸收光谱仪测定其质量分数。
-
数据为3个平行独立试验的平均值±标准差。利用SPSS 26软件进行单因素方差分析(One-way ANOVA)和LSD多重比较(P<0.05)。用Origin 2019b软件绘图,Word 2021制表。
-
如图1所示:八仙花在所有处理下均能存活,即使在2 500 mg·kg−1铅处理下,八仙花也未出现叶片发黄、失水萎蔫甚至整株干枯死亡等重金属毒害症状,但是株高却明显下降。由表1可见:八仙花生物量、根系总长、根系表面积和根尖数随着铅胁迫质量分数的增加而显著降低(P<0.05,表1),2 500 mg·kg−1铅处理下均达到最低,并伴随着根系颜色变褐。耐受指数均大于50%,说明八仙花对铅具有较强的耐受能力。

图 1 铅胁迫对八仙花生长及根系形态的影响
Figure 1. Effects of Pb stress on the growth and root morphology of H. macrophylla
表 1 铅胁迫下八仙花的生长参数
Table 1. The growth parameters of H. macrophylla under Pb stress
铅质量分数/ (mg·kg−1) 生物量/ (g·株−1) 根系总长/cm 根总表面积/cm2 根尖数/个 耐受指数/% 0(ck) 31.14±0.39 a 5 395.37±211.58 a 3 444.48±115.29 a 8 260.62±392.67 a 100.00 a 500 26.10±0.08 b 3 800.38±136.66 b 2 346.04±74.01 b 4 741.27±80.97 b 72.22±0.46 b 1 500 25.17±0.06 c 2 658.66±150.63 c 1 610.68±52.75 c 3 784.25±151.52 c 66.09±0.74 c 2 500 23.77±0.47 d 2 204.41±79.11 d 1 432.78±91.62 d 3 207.75±95.11 d 56.41±0.43 d 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 -
随着土壤铅质量分数的增加,八仙花富集系数、转运系数呈下降趋势,地上及地下部铅质量分数呈上升趋势(表2)。各铅处理的地下部铅质量分数均高于地上部铅质量分数,表明八仙花主要将铅固定在根部。随着土壤铅质量分数的增加,富集系数较对照分别显著降低了27.1%、50.0%和58.3% (P<0.05)。
表 2 铅胁迫下八仙花铅质量分数、富集系数和转运系数
Table 2. Pb concentration, enrichment coefficient and transport coefficient of H. macrophylla under Pb stress
铅质量分数/(mg·kg−1) 地上部铅/(mg·kg−1) 地下部铅/(mg·kg−1) 富集系数 转运系数 0(ck) 2.44±0.06 d 6.24±0.12 d 0.48±0.06 a 0.39±0.02 a 500 14.25±0.13 c 121.33±0.22 c 0.35±0.05 b 0.12±0.01 b 1 500 29.83±0.63 b 256.83±0.38 b 0.24±0.01 c 0.12±0.01 b 2 500 36.00±0.50 a 448.38±1.88 a 0.20±0.01 d 0.10±0.01 c 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 -
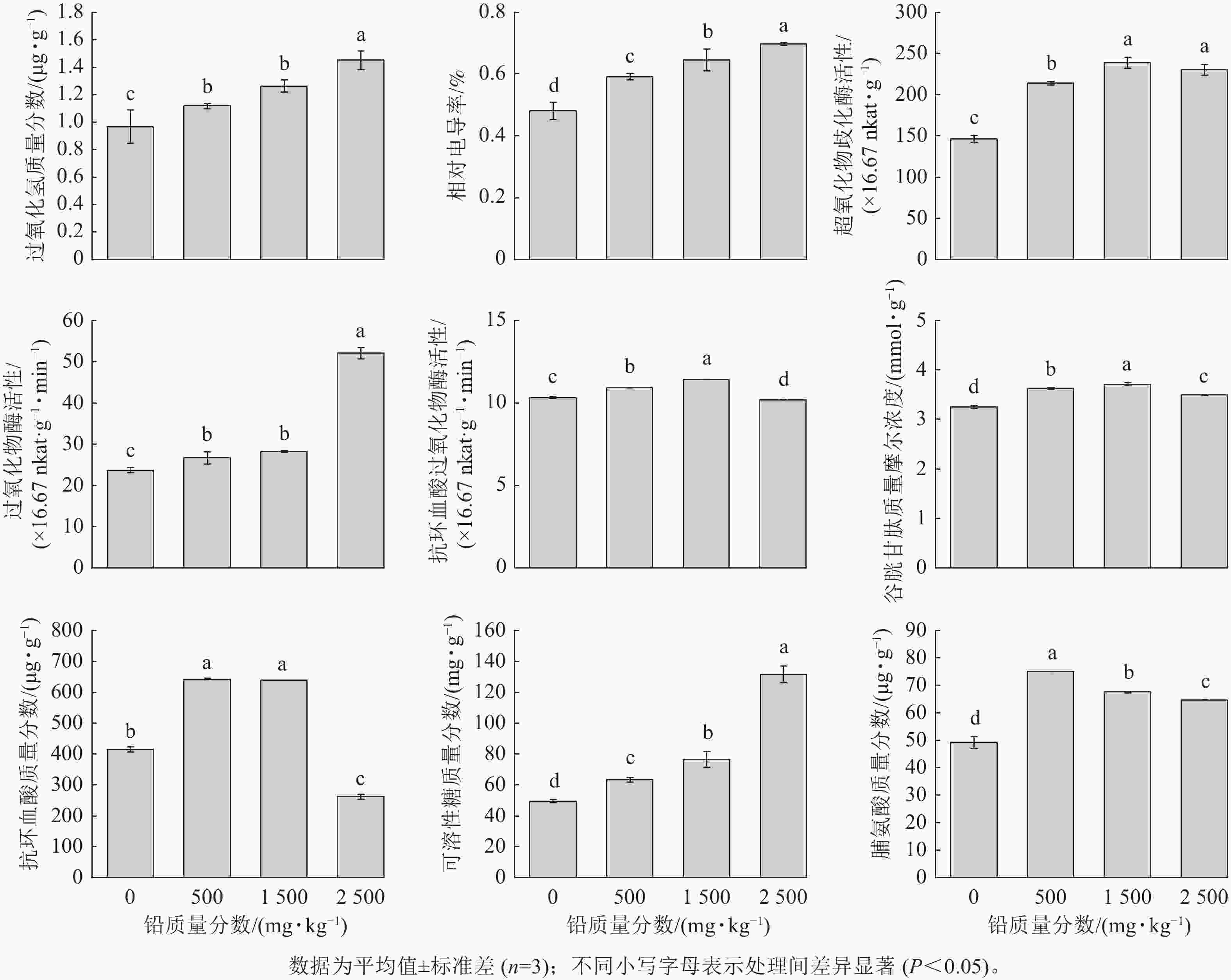
由图2可见,随着铅胁迫质量分数的增加,八仙花根系H2O2质量分数及相对电导率均逐渐上升。表明铅胁迫促使植物体内H2O2积累,对细胞膜造成氧化损伤,使得电解质渗漏增加。八仙花根系中SOD、APX活性及GSH质量摩尔浓度、AsA质量分数随铅质量分数的升高呈先升高后下降的趋势,POD活性随铅质量分数的升高而升高。其中SOD、APX活性及GSH质量摩尔浓度在1 500 mg·kg−1铅处理时最高。AsA质量分数在500、1 500 mg·kg−1铅处理下显著高于对照(P<0.05)。POD活性在2 500 mg·kg−1铅处理时最高,为对照的2.2倍。随着铅胁迫质量分数的增加,可溶性糖质量分数逐渐升高,而游离脯氨酸质量分数则呈先升高后下降的趋势,500 mg·kg−1铅处理时脯氨酸质量分数达到最高。
-
由表3可见:共检测到八仙花根系分泌物249种,其中相对含量最多的分泌物为脂肪酸,最少的为氨基酸。1 500 mg·kg−1铅处理分泌物数量最多(78种)。铅胁迫下八仙花根系分泌脂肪酸相对含量较对照显著上升(P<0.05);有机酸相对含量随铅质量分数的增长呈先上升后降低的趋势,在500 mg·kg−1铅处理下达到最高;氨基酸相对含量在2 500 mg·kg−1铅处理下最高,500、1 500 mg·kg−1铅处理下显著降低(P<0.05);铅胁迫下碳水化合物相对含量显著低于对照(P<0.05);各铅胁迫处理下胺类相对含量显著高于对照(P<0.05);醇类除了1 500 mg·kg−1铅处理下是对照组的69.7%,其他处理下变化不显著。
表 3 铅胁迫对八仙花根系分泌物数量和相对含量的影响
Table 3. Effect of Pb stress on the quantity and relative content of root exudates of H. macrophylla
铅质量分数/
(mg·kg−1)分泌物
数量相对含量/% 脂肪酸 有机酸 氨基酸 碳水化合物 醇类 酯类 胺类 其他 0(ck) 73 25.62±0.69 b 12.33±0.47 b 2.79±0.10 b 9.54±0.06 a 19.38±0.09 a 12.77±0.54 a 6.34±0.30 c 11.23±0.44 a 500 52 25.50±0.32 b 17.28±0.12 a 1.76±0.04 c 8.40±0.10 c 19.82±0.57 a 8.08±0.18 c 13.83±0.65 b 5.68±0.03 c 1 500 78 26.13±0.74 a 11.17±0.51 c 0.85±0.04 d 8.93±0.10 b 13.50±0.13 b 12.32±0.56 a 18.10±0.72 a 8.97±0.75 b 2 500 46 26.83±0.33 a 9.52±0.37 d 6.44±0.48 a 8.95±0.04 b 19.05±0.88 a 9.60±0.34 b 13.43±0.28 b 5.51±0.53 c 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 -
由表4可见:500 mg·kg−1铅处理下土壤pH较对照无显著变化,1 500、2 500 mg·kg−1铅处理下pH显著降低(P<0.05)。土壤EC在500 mg·kg−1铅处理下显著下降(P<0.05),在1 500、2 500 mg·kg−1铅处理下显著上升(P<0.05)。处理组土壤有机质质量分数较对照组分别显著增加了18.6%、18.6%、25.8% (P<0.05)。土壤蔗糖酶和过氧化氢酶活性随土壤铅质量分数的增加而增加,2 500 mg·kg−1铅处理下活性最高。土壤脲酶活性随土壤铅质量分数增加呈先上升后下降的趋势,其中1 500 mg·kg−1铅处理下活性最高,为对照的1.3倍。对照组土壤中残渣态、有机物结合态铅质量分数及占比均达到最大,而500、1 500、2 500 mg·kg−1铅处理下,铁锰氧化物结合态铅质量分数及占比最大。除有机物结合态铅在500 mg·kg−1铅处理下占比与对照相比上升外,残渣态和有机物结合态铅占比随着铅质量分数的增加而呈降低趋势,可交换离子态、碳酸盐结合态铅呈相反趋势。
表 4 铅胁迫对八仙花主要根际特征的影响
Table 4. Effect of Pb stress on the main rhizosphere characteristics of H. macrophylla
铅质量分数/
(mg·kg−1)pH 电导率/
(μS·cm−1)有机质/
(g·kg−1)蔗糖酶活性/
(mg·g−1·d−1)脲酶活性/
(mg·g−1·d−1)过氧化氢酶活性/
(mg·g−1·20 min−1)0(ck) 8.16±0.13 a 126.10±5.58 c 2.85±0.03 c 0.21±0.03 d 114.00±0.82 c 2.01±0.01 d 500 8.21±0.17 a 92.33±3.17 d 3.50±0.27 b 0.30±0.02 c 143.62±1.45 b 2.16±0.05 c 1 500 7.59±0.20 b 173.43±6.92 b 3.50±0.04 b 1.94±0.06 b 151.52±1.40 a 2.44±0.03 b 2 500 7.73±0.15 b 260.33±6.92 a 3.84±0.02 a 2.24±0.04 a 112.91±0.68 c 2.54±0.02 a 铅质量分数/
(mg·kg−1)铅化学形态质量分数及百分比/ (mg·kg−1) 可交换态铅 碳酸盐结合态铅 铁锰氧化物结合态铅 有机物结合态铅 残渣态铅 0(ck) 0 0.13±0.15 (0.53%) 0 8.96±1.36 (35.78%) 15.95±0.14 (63.69%) 500 1.43±0.12 (0.28%) 9.53±0.31 (1.84%) 274.67±12.22 (52.91%) 199.84±5.20 (38.50%) 33.63±3.71 (6.48%) 1 500 17.37±1.63 (1.17%) 74.27±3.79 (5.01%) 933.33±16.65 (62.96%) 416.48±3.39 (28.10%) 40.88±0.88 (2.76%) 2 500 52.67±10.40 (2.43%) 122.35±22.13 (5.66%) 1413.33 ±196.34 (65.34%)524.64 ±13.35 (24.25%)50.08±16.79 (2.32%) 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 -
重金属胁迫对植物生长存在一定的剂量效应,吴桐等[26]发现随着铅质量分数的升高,八仙花的地上干质量呈先增加后下降的趋势。本研究不同质量分数铅胁迫均抑制了八仙花的生长。这是因为重金属胁迫促使植物激活自身防御系统,促进抗氧化酶及抗氧化物质的合成,从而清除体内过量的活性氧,而这一过程需消耗大量能量,故而影响植物的正常生长[27]。重金属胁迫还会阻碍植物根系发育,降低根系发达程度及根系表面活性位点数量,减弱植物从环境中吸收养分和重金属的能力[28]。八仙花对铅有较强的耐受性,通过根部细胞壁吸附和液泡区室化作用,以及膜蛋白严格限制转运,阻断铅向八仙花地上部的流动[11, 29],将铅储存在根部以适应铅污染土壤,提高自身对铅的耐性。
-
H2O2是一种重要的活性氧,反映植物氧化应激水平[30]。外源铅胁迫促使八仙花根系细胞产生过量的H2O2,对细胞膜造成氧化损伤,破坏细胞膜完整性。重金属离子可与细胞膜蛋白、巯基及磷脂类物质结合,使得细胞膜结构损伤、选择性降低及通透性增加,大量电解质渗向细胞外,重金属离子进入胞内,导致细胞内外离子失衡及细胞代谢功能紊乱[31]。
SOD、POD、APX、GSH和AsA是抗氧化系统中主要的抗氧化酶和非酶抗氧化剂,可以有效去除植物体内过量H2O2[32−33],降低重金属对植物的氧化损伤[34]。本研究中铅胁迫下POD活性提高, SOD、APX活性及GSH、AsA水平呈现先上升后下降的趋势。这是因为随铅胁迫强度的增强,细胞中活性氧积累超过限度,使得SOD、APX和AsA分解或失活[33]。2 500 mg·kg−1铅处理下GSH质量分数下降可能因为GSH是合成螯合肽(PCs)的底物,直接与重金属离子结合[34]。
可溶性糖及脯氨酸可以有效防止细胞质渗透势过高造成的细胞脱水和膜脂过氧化,维持细胞代谢活动的正常[20]。有研究表明八仙花叶片中的可溶性糖及脯氨酸质量分数在铅处理下呈上升趋势[10],在八仙花根系中也发现了同一现象,说明可溶性糖及脯氨酸的增加是八仙花应对铅胁迫的自身调节机制。
-
本研究检测到的根系分泌物脂肪酸、醇类和有机酸在八仙花抗铅胁迫中发挥着重要作用。脂肪酸是细胞膜的重要组成成分[35],与植物对重金属胁迫的耐受性呈正相关[16]。1 500和2 500 mg·kg−1铅胁迫显著增加了脂肪酸相对含量。这与SUN等[16]研究结果相反。说明铅胁迫下八仙花通过增加脂肪酸(如棕榈酸)的相对含量,以增加膜的流动性,维持细胞膜的正常结构和功能。植物根系分泌有机酸是一种外部缓解重金属毒性的机制,有机酸能够活化重金属,影响重金属在土壤中的迁移、转化及生物有效性[14]。本研究中500 mg·kg−1铅处理下有机酸相对含量的增加提高了八仙花对铅的吸收能力和耐受能力。这是因为八仙花根系分泌的一些有机酸可以螯合土壤中的铅,增强铅的活动性,从而促进植物对铅的吸收和积累。醇类可以保持细胞的渗透平衡,抵抗胁迫引起的氧化损伤[36]。已有研究表明十八醇相对含量的增加可促进抗氧化酶活性的提升,从而增强植物对重金属的抗性[16]。本研究发现,醇类相对含量只有在1 500 mg·kg−1铅处理下显著降低,说明铅胁迫对八仙花造成了一定的氧化损伤。
根系分泌物中胺类、碳水化合物和氨基酸在应对非生物胁迫时亦发挥着重要作用[37]。胺类相对含量的增加有利于消除活性氧,保护细胞膜,是八仙花对铅胁迫的一种响应机制。铅胁迫下八仙花根系分泌物中碳水化合物比例显著下降,与杜鹃Rhododendron simsii在胁迫下碳水化合物增加不同[36]。这可能因为铅胁迫并未使八仙花将储存在体内的有机营养物通过分解代谢降解,说明八仙花对铅胁迫环境有良好的适应性。高质量分数铅胁迫增加了蛋白质降解,从而增加了氨基酸相对含量。
-
植物根系通过吸收、呼吸和分泌等一系列关键的生物功能改变土壤的生化特性,对土壤-植物系统有重要作用[24]。八仙花根系土壤pH降低是因为重金属Pb2+可以取代土壤中Ca2+、Na+或其他碱性阳离子[38]。此外,植物根系分泌的有机酸也会降低土壤pH[25]。土壤电导率与金属质量分数有关,八仙花根际土壤电导率的增加是由于Pb2+的增加,以及栽培基质湿度波动使得含盐量变高导致的[37]。铅胁迫下有机质比对照组增加。土壤有机质含丰富的官能团,拥有极强的络合能力,可与重金属结合形成难溶沉淀物质,降低重金属在土壤中的迁移性,从而有效降低植物对铅的吸收 [37−38]。
土壤酶活性与土壤养分质量分数密切相关,可指示土壤重金属污染程度[38]。随着铅质量分数的增加,八仙花根际土壤脲酶活性呈先增加后下降的趋势。原因是根系分泌物中含氮有机物(如胺类)增加,提高了土壤中氮质量分数,从而改善土壤肥力和土壤污染状况。而高质量分数铅会抑制土壤中微生物活性[39],且铅会与脲酶活性基团结合,或者与酶底物络合[38],从而抑制脲酶活性。植物根系分泌的低分子量化合物可以促进土壤微生物生长和繁殖,间接促进土壤酶活性的提高[25]。蔗糖酶和过氧化氢酶活性的提高表明了八仙花对铅污染土壤具有修复作用。
可交换离子态、碳酸盐结合态铅在土壤中易发生迁移、转化,具有较高的生物可利用度,易被植物吸收;铁锰氧化物结合态铅可以向可交换离子态、碳酸盐结合态铅转化,具有潜在的生物可利用度;有机物结合态铅可比较稳定存在于土壤中;残渣态铅能长期稳定存在于土壤中,不易于被植物吸收利用[40]。本研究中,铅胁迫下土壤有效态铅质量分数及占比的增加可能与pH降低有关。不同铅胁迫下八仙花根系分泌物组分与质量分数发生变化,使得土壤pH和氧化还原条件发生变化,土壤中高H+浓度可溶解和释放土壤中固定的铅,降低土壤赋存铅的稳定性,促进铅由难溶态向生物可利用态转化,从而有利于植物吸收。
-
铅胁迫抑制了八仙花的生长和铅的富集、转运能力,增加了细胞膜透性,对细胞膜造成氧化损伤,但八仙花对2 500 mg·kg−1铅仍具有一定耐受能力。八仙花根系通过增加抗氧化酶活性(SOD、POD)以及释放更多非酶抗氧化物质(GSH、Pro、SS),缓解铅胁迫造成的氧化损伤,还分泌更多的脂肪酸、胺类,以维持细胞结构、消除活性氧。此外铅胁迫使得土壤有机质及土壤酶活性增加,改善了土壤微生态环境。土壤pH降低、电导率值升高,提升了根际土壤有效态铅的水平,促进了八仙花对铅的吸收。本研究表明八仙花具有极强的铅耐受能力,是用于铅污染土壤修复的理想植物,未来可通过外源添加根系分泌的特定物质、改善根际特征来提高八仙花修复效率。
Effects of Pb stress on physiology and main rhizosphere characteristics of Hydrangea macrophylla
-
摘要:
目的 探究铅胁迫对八仙花Hydrangea macrophylla根系生长生理、根系分泌物和根际土壤养分环境的影响,分析八仙花对铅胁迫的适应机制 ,为八仙花修复铅污染土壤提供科学依据。 方法 通过50 d的温室盆栽试验,研究0 (对照)、500、1 500、2 500 mg·kg−1铅胁迫对八仙花生长和铅吸收,根系氧化损伤、抗氧化系统、渗透调节物质和根系分泌物,及土壤理化性质、土壤酶和根际铅化学形态的影响。 结果 铅胁迫抑制了八仙花生长,尤其在2 500 mg·kg−1铅处理下,八仙花生物量较对照显著降低23.7%。但八仙花对高质量分数铅有一定耐受性,八仙花通过提高根系超氧化物歧化酶(SOD)和过氧化物酶(POD)活性,增加谷胱甘肽(GSH)质量分数,增强抗氧化能力,从而缓解铅胁迫对八仙花造成的氧化损伤;铅胁迫还提高了八仙花渗透调节物质的质量分数,其中可溶性糖质量分数在2 500 mg·kg−1铅处理下达到最大,脯氨酸质量分数则相反;八仙花根系可以分泌脂肪酸、胺类,维持细胞结构、消除活性氧,乙醇酸分泌的增加促进了对铅的吸收;铅胁迫下土壤微生态环境得到改善,根际土壤蔗糖酶和过氧化氢酶活性在2 500 mg·kg−1铅处理下达到最大,有效提高了土壤养分;随着铅质量分数的升高,根际pH降低、电导率增加,土壤铅可交换离子态、碳酸盐结合态占比最高分别达到2.03%、8.09%,提升了土壤铅的有效态成分,有利于八仙花对铅的吸收。 结论 八仙花对铅有良好的适应性,能够在含高质量分数铅的土壤中正常生长并改善土壤理化性质。图2表4参40 Abstract:Objective The objective is to explore the effects of different concentrations of lead (Pb) stress on the root growth physiology, root exudates and rhizosphere soil nutrient environment of Hydrangea macrophylla, and analyze its adaptation mechanism, provide a scientific basis to repair lead-contaminated soil. Method The effects of Pb [0 (the control), 500, 1 500, 2 500 mg·kg−1] stress on the growth and uptake of Pb, root oxidative damage, root antioxidant system, root osmotic regulatory substances and root exudates, soil physical and chemical properties, soil enzymes and rhizosphere Pb chemical morphology were studied by a pot experiment. Result Pb stress inhibited the growth of H. macrophylla, especially under 2 500 mg·kg−1 Pb treatment, the biomass decreased significantly by 23.7% compared with the control, but H. macrophylla had a certain tolerance to high concentrations of Pb. The oxidative damage caused by Pb stress on H. macrophylla was alleviated by increasing SOD and POD activities in the root, increasing GSH content and enhancing the antioxidant capacity. H. macrophylla also increased the content of osmotic regulatory substances. The soluble sugar content reached the maximum under 2 500 mg·kg−1 Pb treatment, while the proline content was opposite. The root could secrete fatty acids and amines to maintain cell structure, eliminate reactive oxygen species, and the increase of glycolic acid secretion promoted Pb absorption. Soil microecological environment was improved under Pb stress. The activities of sucrase and catalase in rhizosphere soil reached the maximum under the treatment of 2500 mg·kg−1 Pb, which effectively improved soil nutrients. With the increase of Pb concentration, rhizosphere pH value decreased and EC value increased, and the exchangeable ionic state and carbonate binding state of soil Pb accounted for 2.03% and 8.09% respectively, which increased the available state composition of Pb and was conducive to the absorption of Pb by H. macrophylla.Conclusion H. macrophylla has good adaptability to Pb, and can grow normally in soil with high concentration of Pb and improve the physical and chemical properties of soil. [Ch, 2 fig. 4 tab. 40 ref.] -
表 1 铅胁迫下八仙花的生长参数
Table 1. The growth parameters of H. macrophylla under Pb stress
铅质量分数/ (mg·kg−1) 生物量/ (g·株−1) 根系总长/cm 根总表面积/cm2 根尖数/个 耐受指数/% 0(ck) 31.14±0.39 a 5 395.37±211.58 a 3 444.48±115.29 a 8 260.62±392.67 a 100.00 a 500 26.10±0.08 b 3 800.38±136.66 b 2 346.04±74.01 b 4 741.27±80.97 b 72.22±0.46 b 1 500 25.17±0.06 c 2 658.66±150.63 c 1 610.68±52.75 c 3 784.25±151.52 c 66.09±0.74 c 2 500 23.77±0.47 d 2 204.41±79.11 d 1 432.78±91.62 d 3 207.75±95.11 d 56.41±0.43 d 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 表 2 铅胁迫下八仙花铅质量分数、富集系数和转运系数
Table 2. Pb concentration, enrichment coefficient and transport coefficient of H. macrophylla under Pb stress
铅质量分数/(mg·kg−1) 地上部铅/(mg·kg−1) 地下部铅/(mg·kg−1) 富集系数 转运系数 0(ck) 2.44±0.06 d 6.24±0.12 d 0.48±0.06 a 0.39±0.02 a 500 14.25±0.13 c 121.33±0.22 c 0.35±0.05 b 0.12±0.01 b 1 500 29.83±0.63 b 256.83±0.38 b 0.24±0.01 c 0.12±0.01 b 2 500 36.00±0.50 a 448.38±1.88 a 0.20±0.01 d 0.10±0.01 c 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 表 3 铅胁迫对八仙花根系分泌物数量和相对含量的影响
Table 3. Effect of Pb stress on the quantity and relative content of root exudates of H. macrophylla
铅质量分数/
(mg·kg−1)分泌物
数量相对含量/% 脂肪酸 有机酸 氨基酸 碳水化合物 醇类 酯类 胺类 其他 0(ck) 73 25.62±0.69 b 12.33±0.47 b 2.79±0.10 b 9.54±0.06 a 19.38±0.09 a 12.77±0.54 a 6.34±0.30 c 11.23±0.44 a 500 52 25.50±0.32 b 17.28±0.12 a 1.76±0.04 c 8.40±0.10 c 19.82±0.57 a 8.08±0.18 c 13.83±0.65 b 5.68±0.03 c 1 500 78 26.13±0.74 a 11.17±0.51 c 0.85±0.04 d 8.93±0.10 b 13.50±0.13 b 12.32±0.56 a 18.10±0.72 a 8.97±0.75 b 2 500 46 26.83±0.33 a 9.52±0.37 d 6.44±0.48 a 8.95±0.04 b 19.05±0.88 a 9.60±0.34 b 13.43±0.28 b 5.51±0.53 c 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 表 4 铅胁迫对八仙花主要根际特征的影响
Table 4. Effect of Pb stress on the main rhizosphere characteristics of H. macrophylla
铅质量分数/
(mg·kg−1)pH 电导率/
(μS·cm−1)有机质/
(g·kg−1)蔗糖酶活性/
(mg·g−1·d−1)脲酶活性/
(mg·g−1·d−1)过氧化氢酶活性/
(mg·g−1·20 min−1)0(ck) 8.16±0.13 a 126.10±5.58 c 2.85±0.03 c 0.21±0.03 d 114.00±0.82 c 2.01±0.01 d 500 8.21±0.17 a 92.33±3.17 d 3.50±0.27 b 0.30±0.02 c 143.62±1.45 b 2.16±0.05 c 1 500 7.59±0.20 b 173.43±6.92 b 3.50±0.04 b 1.94±0.06 b 151.52±1.40 a 2.44±0.03 b 2 500 7.73±0.15 b 260.33±6.92 a 3.84±0.02 a 2.24±0.04 a 112.91±0.68 c 2.54±0.02 a 铅质量分数/
(mg·kg−1)铅化学形态质量分数及百分比/ (mg·kg−1) 可交换态铅 碳酸盐结合态铅 铁锰氧化物结合态铅 有机物结合态铅 残渣态铅 0(ck) 0 0.13±0.15 (0.53%) 0 8.96±1.36 (35.78%) 15.95±0.14 (63.69%) 500 1.43±0.12 (0.28%) 9.53±0.31 (1.84%) 274.67±12.22 (52.91%) 199.84±5.20 (38.50%) 33.63±3.71 (6.48%) 1 500 17.37±1.63 (1.17%) 74.27±3.79 (5.01%) 933.33±16.65 (62.96%) 416.48±3.39 (28.10%) 40.88±0.88 (2.76%) 2 500 52.67±10.40 (2.43%) 122.35±22.13 (5.66%) 1413.33 ±196.34 (65.34%)524.64 ±13.35 (24.25%)50.08±16.79 (2.32%) 说明:数据为平均值±标准差(n=3);同列不同小写字母表示不同处理间差异显著(P<0.05)。 -
[1] 顾绍茹, 杨兴, 陈翰博, 等. 小龙虾壳炭和细叶榕枝条炭对土壤养分及镉和铅生物有效性的影响[J]. 浙江农林大学学报, 2023, 40(1): 176−187. GU Shaoru, YANG Xing, CHEN Hanbo, et al. Effects of biochar from Procambarus clarkii shells and Ficus microcarpa branches on soil nutrients and bioavailability of Cd and Pb [J]. Journal of Zhejiang A&F University, 2023, 40(1): 176−187. [2] 颜越, 金荷仙, 王瑛, 等. 间作模式对社区花园可食植物生理特性及铅吸收的影响[J]. 浙江农林大学学报, 2023, 40(2): 338−347. YAN Yue, JIN Hexian, WANG Ying, et al. Effects of intercropping patterns on physiological characteristics and Pb uptake of edible plants in community gardens [J]. Journal of Zhejiang A&F University, 2023, 40(2): 338−347. [3] KAUSHAL J, MAHAJAN P, KAUR N. A review on application of phytoremediation technique for eradication of synthetic dyes by using ornamental plants [J]. Environmental Science and Pollution Research, 2021, 28(48): 67970−67989. [4] ROCHA C S, ROCHA D C, KOCHI L Y, et al. Phytoremediation by ornamental plants: a beautiful and ecological alternative [J]. Environmental Science and Pollution Research, 2022, 29: 3336−3354. [5] CAPUANA M. A review of the performance of woody and herbaceous ornamental plants for phytoremediation in urban areas [J]. iForest-Biogeosciences and Forestry, 2020, 13(1): 139−151. [6] 赵冰. 中国八仙花[M]. 北京: 中国林业出版社, 2016. ZHAO Bing. Chinese Hydrangea [M]. Beijing: China Forestry Publishing House, 2016. [7] DUAN Yaping, ZHANG Ying, ZHAO Bing. Lead, zinc tolerance mechanism and phytoremediation potential of Alcea rosea (Linn. ) Cavan. and Hydrangea macrophylla (Thunb. ) Ser. and ethylenediaminetetraacetic acid effect [J]. Environmental Science and Pollution Research, 2022, 29(27): 41329−41343. [8] MA Wenjie, ZHAO Bing, LÜ Xiaofan, et al. Lead tolerance and accumulation characteristics of three Hydrangea cultivars representing potential lead-contaminated phytoremediation plants [J]. Horticulture, Environment, and Biotechnology, 2022, 63(1): 23−38. [9] 邢春艳, 周玉卿, 赵九洲, 等. 野生圆锥八仙花对Pb(NO3)2重金属胁迫的生长及生理响应[J]. 北方园艺, 2020(18): 71−77. XING Chunyan, ZHOU Yuqing, ZHAO Jiuzhou, et al. Growth and physiological response of Hydrangea paniculata Sieb to Pb(NO3)2 heavy metal stress [J]. Northern Horticulture, 2020(18): 71−77. [10] ZHANG Yuyu, SONG Ziyi, ZHAO Huiqi, et al. Integrative physiological, transcriptomic and metabolomic analysis reveals how the roots of two ornamental Hydrangea macrophylla cultivars cope with lead (Pb) toxicity [J/OL]. Science of the Total Environment, 2024, 910 : 168615[2024-04-01]. DOI: 10.1016/j.scitotenv.2023.168615. [11] 张瑛, 宋子怡, 赵冰. 八仙花对铅锌的抗性及富集特征[J]. 东北林业大学学报, 2022, 50(8): 41−48. ZHANG Ying, SONG Ziyi, ZHAO Bing. Lead and zinc tolerance mechanisms and accumulation characteristics of Hydrangea macrophylla [J]. Journal of Northeast Forestry University, 2022, 50(8): 41−48. [12] JIN Jing, SONG Ziyi, ZHAO Bing, et al. Physiological and metabolomics responses of Hydrangea macrophylla (Thunb. ) Ser. and Hydrangea strigosa Rehd. to lead exposure [J/OL]. Ecotoxicology and Environmental Safety, 2022, 243 : 113960[2024-04-01]. DOI: 10.1016/j.ecoenv.2022.113960. [13] LIN Hai, LIN Chenjing, LI Bing, et al. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms [J/OL]. Journal of Hazardous Materials, 2021, 402 : 123829[2024-04-01]. DOI: 10.1016/j.jhazmat.2020.123829. [14] 徐炜杰, 郭佳, 赵敏, 等. 重金属污染土壤植物根系分泌物研究进展[J]. 浙江农林大学学报, 2017, 34(6): 1137−1148. XU Weijie, GUO Jia, ZHAO Min, et al. Research progress of soil plant root exudates in heavy metal contaminated soil [J]. Journal of Zhejiang A&F University, 2017, 34(6): 1137−1148. [15] KHAN N, ALI S, SHAHID M A, et al. Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: a review [J/OL]. Cells, 2021, 10 (6): 1551[2024-04-01]. DOI: 10.3390/cells10061551. [16] SUN Lijuan, CAO Xueying, TAN Changyin, et al. Analysis of the effect of cadmium stress on root exudates of Sedum plumbizincicola based on metabolomics [J]. Ecotoxicology and Environmental Safety, 2020, 205 : 9[2024-04-01]. DOI: 10.1016/j.ecoenv.2020.111152. [17] 段亚萍, 赵冰, 付丽童, 等. 铅、锌污染下蜀葵的生长生理响应和富集转运特性研究[J]. 草地学报, 2022, 30(2): 418−425. DUAN Yaping, ZHAO Bing, FU Litong, et al. Study on growth, physiological response and accumulation, transport characteristics of Alcea rosea (Linn. ) Cavan. under lead and zinc pollution [J]. Acta Agrestia Sinica, 2022, 30(2): 418−425. [18] 刘楚藩, 肖荣波, 黄飞, 等. 土壤与大气双重胁迫下苋菜幼苗对铅的累积与生理响应[J]. 生态学报, 2020, 40(24): 9174−9183. LIU Chufan, XIAO Rongbo, HUANG Fei, et al. Accumulation and physiological response of Amaranthus tricolor L. seedings to lead under soil and atmospheric stress [J]. Acta Ecologica Sinica, 2020, 40(24): 9174−9183. [19] 姚虹宇, 刘亚敏, 张盛楠, 等. 外源柠檬酸对铝胁迫下马尾松生理特性的影响[J]. 林业科学, 2018, 54(7): 155−164. YAO Hongyu, LIU Yamin, ZHANG Shengnan, et al. Effects of exogenous citric acid on physiological characteristics of Pinus massoniana under aluminum stress [J]. Scientia Silvae Sinicae, 2018, 54(7): 155−164. [20] 安锋, 李昌珍, 张婷婷, 等. 铝胁迫对橡胶苗生理和叶绿素荧光特性的影响[J]. 应用生态学报, 2018, 29(12): 4191−4198. AN Feng, LI Changzhen, ZHANG Tingting, et al. Effects of aluminum toxicity on physiological and leaf chlorophyll fluorescent characteristics of rubber tree seedlings [J]. Chinese Journal of Applied Ecology, 2018, 29(12): 4191−4198. [21] 韩一林, 王鑫朝, 许馨露, 等. 毛竹幼苗抗氧化酶和AsA-GSH循环对高温干旱及协同胁迫的响应[J]. 浙江农林大学学报, 2018, 35(2): 268−276. HAN Yilin, WANG Xinzhao, XU Xinlu, et al. Responses of anti-oxidant enzymes and the ascorbate-glutathione cycle to heat, drought, and synergistic stress in Phyllostachys edulis seedlings [J]. Journal of Zhejiang A&F University, 2018, 35(2): 268−276. [22] MAZUMDAR K, DAS S. Phytoremediation of soil treated with metalliferous leachate from an abandoned industrial site by Alternanthera sessilis and Ipomoea aquatica: metal extraction and biochemical responses [J/OL]. Ecological Engineering, 2021, 170 : 106349[2024-04-01]. DOI: 10.1016/j.ecoleng.2021.106349. [23] ZHANG Ying, JIANG Duo, YANG Chao, et al. The oxidative stress caused by atrazine in root exudation of Pennisetum americanum (L. ) K. Schum [J/OL]. Ecotoxicology and Environmental Safety, 2021, 211 : 111943[2024-04-01]. DOI: 10.1016/j.ecoenv.2021.111943. [24] 高凤, 杨凤军, 吴瑕, 等. 施用生物炭对白菜根际土壤中有机质含量及酶活性的影响[J]. 土壤通报, 2019, 50(1): 103−108. GAO Feng, YANG Fengjun, WU Xia, et al. Effects of biochar application on organic matter content and enzyme activity in rhizosphere soil of Chinese cabbage [J]. Chinese Journal of Soil Science, 2019, 50(1): 103−108. [25] 易艳灵, 吴丽英, 杨倩, 等. 柏木根系分泌物对盆栽香椿土壤养分和酶活性的影响[J]. 生态学杂志, 2019, 38(7): 2080−2086. YI Yanling, WU Liying, YANG Qian, et al. Effects of root exudates of Cupressus funebris on soil nutrients and enzyme activities of potted Toona sinensis [J]. Chinese Journal of Ecology, 2019, 38(7): 2080−2086. [26] 吴桐, 李翠兰, 邵泽强, 等. 几种花卉植物对土壤中铅富集特征的研究[J]. 吉林农业大学学报, 2012, 34(3): 305−310, 315. WU Tong, LI Cuilan, SHAO Zeqiang, et al. Enrichment characteristics of several ornamental plants to lead in soil [J]. Journal of Jilin Agricultural University, 2012, 34(3): 305−310, 315. [27] 冯嘉仪, 阮可瑾, 苏思宁, 等. 构树的污泥适应性及养分和重金属吸收累积特征[J]. 应用生态学报, 2022, 33(6): 1629−1638. FENG Jiayi, Ruan Kejin, SU Sining, et al. Adaptability of Broussonetia papyrifera to sewage sludge and its characteristics of nutrient and heavy metal uptake and accumulation [J]. Chinese Journal of Applied Ecology, 2022, 33(6): 1629−1638. [28] 王效瑾, 高巍, 赵鹏, 等. 小麦幼苗根系形态对镉胁迫的响应[J]. 农业环境科学学报, 2019, 38(6): 1218−1225. WANG Xiaojin, GAO Wei, ZHAO Peng, et al. Changes to wheat seedling root morphology in response to cadmium stress [J]. Journal of Agro-Environment Science, 2019, 38(6): 1218−1225. [29] FORTE J, MUTITI S. Phytoremediation potential of Helianthus annuus and Hydrangea paniculata in copper and lead-contaminated soil [J]. Water, Air, & Soil Pollution, 2017, 228 (2): 1−11. [30] ABID R, MANZOOR M, DE OLIVEIRA L M, et al. Interactive effects of As, Cd and Zn on their uptake and oxidative stress in As-hyperaccumulator Pteris vittate [J]. Environmental Pollution, 2019, 248: 756−762. [31] 谢倚慧, 张明华, 熊瑞, 等. 马缨丹在镉、铅、锌复合胁迫下的耐性和解毒机制[J]. 生态与农村环境学报, 2021, 37(9): 1209−1217. XIE Yihui, ZHANG Minghua, XIONG Rui, et al. Study on the tolerance and detoxification mechanisms of lantana camara under the combined stress of Cadmium, Lead and Zinc [J]. Journal of Ecology and Rural Environment, 2021, 37(9): 1209−1217. [32] 徐圆圆, 陆明英, 蒋维昕, 等. 铝胁迫下不同耐铝型桉树无性系根和叶抗氧化特征的差异[J]. 浙江农林大学学报, 2016, 33(6): 1009−1016. XU Yuanyuan, LU Mingying, JIANG Weixin, et al. Al stress with lipid peroxidation and antioxidant enzyme activities in eucalyptus roots and leaves [J]. Journal of Zhejiang A&F University, 2016, 33(6): 1009−1016. [33] 孙海燕, 孔德庸, 胡慧影, 等. 腐植酸浸种对低温胁迫下玉米幼苗抗氧化系统的影响[J]. 生态学报, 2021, 41(13): 5385−5397. SUN Haiyan, KONG Deyong, HU Huiying, et al. Effects of soaking seed with humic acid on antioxidant system of maize seedlings under low temperature stress [J]. Acta Ecologica Sinica, 2021, 41(13): 5385−5397. [34] 全芮萍, 陈建福, 张蕾, 等. 抗氧化酶和植物螯合肽对苎麻重金属Cd胁迫的应答[J]. 热带作物学报, 2022, 43(5): 1023−1031. QUAN Ruiping, CHEN Jianfu, ZHANG Lei, et al. Responses of ramie to antioxidant enzymes and plant chelating peptides to Cd stress [J]. Chinese Journal of Tropical Crops, 2022, 43(5): 1023−1031. [35] FERRERI C, MASI A, SANSONE A, et al. Fatty acids in membranes as homeostatic, metabolic and nutritional biomarkers: recent advancements in analytics and diagnostics [J/OL]. Diagnostics, 2017, 7 (1): 1[2024-04-01]. DOI: 10.3390/diagnostics7010001. [36] WANG Jiaolong, FAROOQ T H, ASLAM A, et al. Non-targeted metabolomics reveal the impact of phenanthrene stress on root exudates of ten urban greening tree species [J/OL]. Environmental Research, 2021, 196 : 110370[2024-04-01]. DOI: 10.1016/j.envres.2020.110370. [37] KUMAR P, FULEKAR M H. Cadmium phytoremediation potential of Deenanath grass (Pennisetum pedicellatum) and the assessment of bacterial communities in the rhizospheric soil [J]. Environmental Science and Pollution Research, 2022, 29(2): 2936−2953. [38] WU Bohan, LUO Shihua, LUO Huanyan, et al. Improved phytoremediation of heavy metal contaminated soils by Miscanthus floridulus under a varied rhizosphere ecological characteristic [J/OL]. Science of the Total Environment, 2022, 808 : 151995[2024-04-01]. DOI: 10.1016/j.scitotenv.2021.151995. [39] YANG Yan, SHEN Qianyong. Phytoremediation of cadmium-contaminated wetland soil with Typha latifolia L. and the underlying mechanisms involved in the heavy-metal uptake and removal [J]. Environmental Science and Pollution Research, 2020, 27(5): 4905−4916. [40] 李娜, 夏瑜, 何绪文, 等. 基于Tessier法的土壤中不同形态镉的转化及其影响因素研究进展[J]. 土壤通报, 2021, 52(6): 1505−1512. LI Na, XIA Yu, HE Xuwen, et al. Research progress of cd form transformation and the effective environmental factors in soil based on Tessier analysis [J]. Chinese Journal of Soil Science, 2021, 52(6): 1505−1512. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20240301






 下载:
下载: