-
维生素B6(VB6)是一类吡啶化合物的总称,包括吡哆醛(PL),吡哆醇(PN),吡哆胺(PM),磷酸吡哆醛(PLP),磷酸吡哆醇(PNP),磷酸吡哆胺(PMP)等,其中PLP是其主要活性形式,作为辅酶参与生物体内100多种生化反应,包括氨基酸代谢、抗生素合成、免疫调节等生理反应及氧化胁迫等抗逆反应[1]。细胞内VB6各组分的平衡是机体进行正常代谢的前提,因而VB6对植物的生长发育至关重要。研究[2]发现,自然界中VB6有从头合成(de novo synthetic pathway)和补救合成(salvage pathway)2种方式,补救合成途径使VB6异养型生物能够利用外源摄入的PN,PM和PL来合成机体代谢所需要的活化型PLP并维持细胞各型VB6浓度相对稳定。从头合成已被广泛研究,而补救合成途径的研究却相对缺乏。其中,吡哆醛还原酶(PLR)是VB6补救合成途径中的作用酶,最初在酵母中被发现,属于醛酮还原酶(aldo-keto reductase),在还原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)存在的条件下催化PL转换成PN[3-4],从而可维持细胞内VB6动态平衡,对于生物体进行正常的生理活动具有重要意义。植物性食物中VB6主要以PN或其糖基化形态存在,并推测植物中可能有高效的PN生成机制[5]。迄今为止,植物中只有拟南芥Arabidopsis thaliana的AtPLR1基因得到分离鉴定,对酵母突变体进行互补实验表明AtPLR1像酵母PLR一样,可催化PL形成PN[6]。T-DNA插入的Atplr1突变体根系生长较野生型明显缓慢,氯化钠、甘露醇胁迫下Atplr1的生长受到抑制[6],推测PLR可能与植物抵抗盐害和渗透压胁迫有关。烟草Nicotiana tabacum是一种重要的模式植物及经济作物,对其PLR进行克隆和功能分析,有助于进一步明确植物体内VB6的补救合成途径,同时为烟草良种选育提供理论储备。本研究以AtPLR1为模板,经过对美国生物技术信息中心(NCBI)公共数据库(http://www.ncbi.nlm.nih.gov/)中的序列进行比对和拼接并结合cDNA末端快速扩增技术-聚合酶链式反应(RACE-PCR)得到了烟草NtPLR1基因的全长序列。以此为基础,分析了其生物功能和表达特性,结果NtPLR1可催化PL形成PN。NtPLR1在叶片中表达最高,与紫外线、氧化及氯化钠胁迫和外源PL处理有应答反应。
-
烟草‘云烟85’Nicotiana tabacum‘Yunyan 85’种子和pET32a原核表达载体为实验室保存。pEASY-Blunt载体、菌株BL21(DE3)Rosetta和大肠埃希菌Escherichia coli DH5α购自TransGen公司。
-
紫外线处理:在无菌操作台上用紫外线照射生长至旺长期的烟草,照射时间分别为2 h,4 h和8 h,取茎尖以下的第3片叶,液氮冷冻,备用。
盐处理:用100.0 mmol·L-1的氯化钠溶液浇灌旺长期烟草,处理1 d,4 d和7 d后取样,取茎尖以下第2片叶。液氮冷冻,备用。
氧化处理:浇灌亚硫酸氢钠-亚硫酸钠(NaHSO3-Na2SO3)混合物(10.0 mmol·L-1,以亚硫酸钠浓度计),处理1 d,4 d和7 d后取样,取茎尖以下第2片叶,液氮冷冻,备用。
PL处理:将旺长期烟草根部洗净,置于添加100.0 mg·L-1 PL的水培液中,用锡纸将烟草根部及水培液遮住,茎叶接受正常光照。分别于培养的第2天、第4天和第8天采集茎尖以下第2片叶,液氮冷冻,备用。
以上均设置重复3个·处理-1。
-
根据RNAiso Plus RNA提取试剂盒使用说明书(Takara),进行总RNA提取。提取的RNA用质量分数为1.0%琼脂糖凝胶电泳进行纯度与完整性检测。参照PrimeScript RT reagent Kit with gDNA Eraser反转录试剂盒(Takara)对质量合格的RNA进行反转录,置于-20 ℃备用。
-
以拟南芥吡哆醛PLR的氨基酸序列(NP_200170.2)为模板,在烟草表达序列标签(EST)数据库里同源检索,根据得到的EST序列,设计引物3RACE-1,3RACE-2,3RACE-3(表 1)进行巢式PCR扩增目的基因3′端序列。扩增产物经切胶回收,连接pEASY-Blunt载体后,转化至大肠埃希菌DH5α感受态细胞,筛选阳性克隆子进行测序。3′端序列扩增产物测序结果验证后,使用DNAMAN软件将它与EST起始序列结合得到全长cDNA序列。随后设计全长克隆引物NtPLR-F1和NtPLR-R1(表 1),扩增NtPLR1序列。PCR反应程序为95 ℃预变性3.0 min;94 ℃变性30 s,60 ℃退火30 s,72 ℃延伸1.5 min,共28个循环;72 ℃延伸10.0 min。获得的NtPLR1序列在NCBI(http://www.ncbi.nlm.nih.gov/)数据库BLAST中进行序列比对。用ProtParam软件(http://web.expasy.org/protparam/)在线分析该蛋白的分子量和等电点;采用DNAMAN 7.0软件对基因编码的氨基酸序列进行比对分析。
表 1 NtPLR1基因克隆与表达分析所用引物信息
Table 1. Primers used in NtPLR1 gene cloning and expression analysis
用途 引物名称 引物序列(5'→3') 3'-RACE 3RACE-1 TGCAAATTATGCACCTCTGCAGGAACG 3RACE-2 TGCAGTTGGGGTGAGCAACTATGGACC 3RACE-3 TGCGCTCAGCCCAGGTACAATTTTCAT 目的片段扩增NtPLR1 NtPLR-F1 ATGGCTCTCTCACTCCCAGCTTCAAAATC NtPLR-R1 CTTTGTCTGAAATACGTTTTGGATC 原核表达 NtPLR-F2 GGATCCATGGCTCTCTCACTCCCAGCTTCAAAATC NtPLR-R2 GTCGACCTTTGTCTGAAATACGTTTTGGATC qRT-PCR NtPLR-F3 TGGCAAAAGGTAAAGATGGG NtPLR-R3 GTTGATGCCATTCTCCACCG 说明:下划线分别表示BamH1和Sal1。 -
根据NtPLR1基因的cDNA序列,设计特异性定量引物(表 1),以18 S rRNA为内参基因,以根、茎、叶及紫外线、氧化、氯化钠处理下不同时间点取样叶片的cDNA为模板,进行荧光实时定量PCR(qRT-PCR)分析。qRT-PCR反应程序为95 ℃ 3.0 min,95 ℃ 10 s,55 ℃ 40 s,35个循环;溶解曲线:从65 ℃按0.5 ℃/循环增加到95 ℃。以2-ΔΔCt法计算相对表达量。
-
VB6检测参照张剑韵等[7-9]的方法,加以改进。VB6色谱分析所用色谱柱为H&E公司的XP ODS-A 5 μm 120 A(250.0 mm × 4.6 mm)。高效液相色谱仪为Waters 600,配备2475荧光检测仪。流动相A(分析用):体积分数为1%乙腈(CH3CN)-25.0 mmol·L-1磷酸二氢钾(KH2PO4)-25.0 mmol·L-1高氯酸钠(NaClO4),pH 2.5;流速为0.5 mL·min-1。进样量均为5.0 μL,荧光检测波长为395 nm,调整激发波长为290 nm。
-
原核表达载体构建:根据载体pET32a多克隆位点信息,设计带有酶切位点(BamH1和Sal1)的原核表达引物(表 1),以云烟85 cDNA为模板扩增NtPLR1基因的cDNA片段。经琼脂糖凝胶电泳分离,回收目的条带后,连接pEASY-Blunt载体,转化至大肠埃希菌DH5α感受态细胞。挑取经PCR和测序验证的阳性菌落,扩大培养,使用质粒小抽试剂盒(TransGen2)提取质粒,即得到pEASY-NtPLR1载体。用限制性内切酶BamH1和Sal1双酶切pEASY-NtPLR1和pET32a质粒后进行T4连接,即得到pET32a-NtPLR1重组质粒。测序正确后,提取目的质粒并转化至BL21(DE3)Rosetta菌株,即得到融合表达菌。
蛋白诱导表达:37 ℃培养融合表达菌至D(600)约为0.60,加入终浓度为1.0 mmol·L-1的异丙基硫代半乳糖苷(IPTG),并在18 ℃/37 ℃ 200 r·min-1诱导24 h。以含pET32a空质粒的BL21(DE3)Rosetta为对照。离心收获菌体,8 000 r·min-1离心10.0 min,弃上清,用PBS重悬。重复1次后进行超声波破碎。分别取上清和沉淀,加入上样缓冲液,沸水浴5.0 min,冷却至室温后,取20.0 μL进行SDS-PAGE(5%浓缩胶,10%分离胶)电泳检测。电泳后,经考马斯亮蓝染色、拍照,分析蛋白表达结果。
-
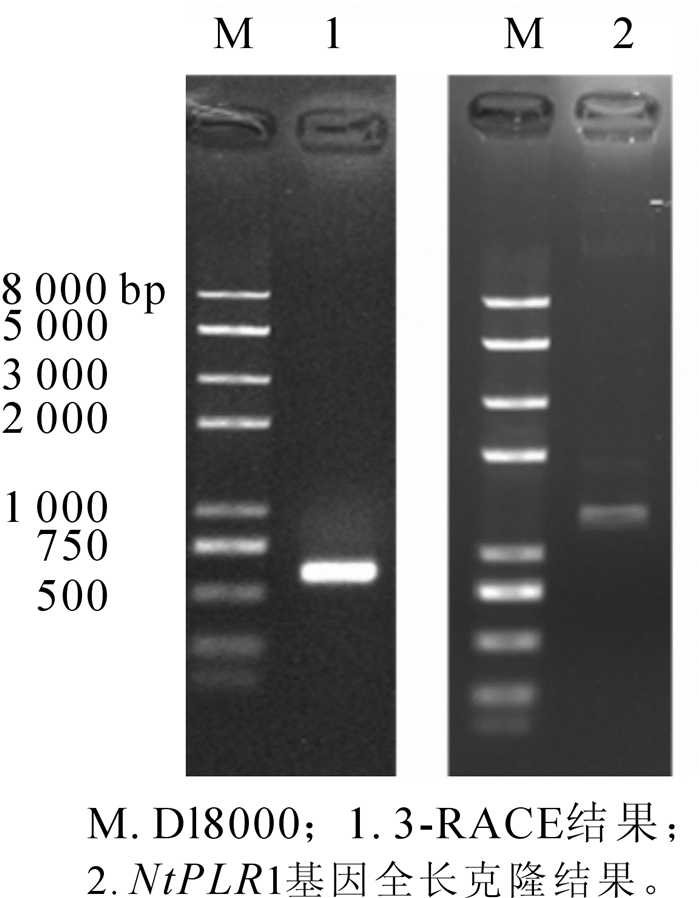
以拟南芥吡哆醛PLR的氨基酸序列(NP_200170.2)为模板,在烟草EST数据库里同源检索到一条同源性(79%)序列(GenBank: HS082453.1)。以此EST序列为起点,进行延伸检索后得到4条候选EST序列,登录号分别为GenBank:FS425789,GenBank:FS385536.1,GenBank:FS432618,GenBank:FS431044.1。使用DNAMAN比对5条EST序列,发现它们来源于同一基因,拼接后得到1条长800 bp的起始序列。根据该序列设计3轮3′-RACE引物(表 1),进行巢式PCR扩增得到大小约为700 bp单一明亮条带(图 1中泳道1)。将该条带测序后和起始序列拼接得到全长cDNA序列。随后设计全长克隆引物(表 1),PCR扩增得到大小约1 500 bp的序列(图 1中泳道2)。测序正确后,将此全长序列命名为NtPLR1,其cDNA长度为1 370 bp,开放阅读框1 110 bp,5′非编码区(UTR)长55 bp,3′UTR长205 bp。编码369个氨基酸,起始密码子为ATG,终止密码子为TGA(图 2),具有Aldo-keto还原酶家族保守底物结合位点[6](图 2中下划线强调部分)。在线预测其编码蛋白的分子量为41 070.5 Da,理论等电点为9.42。氨基酸多序列比对结果显示,NtPLR1与AtPLR相似性为75%(图 3),与栗酒裂殖酵母Schizosaccharomyces pombe[10],酿酒酵母Saccharomyces cerevisiae[11]PLR的氨基酸相似性分别为24%和26%。
-
分别提取烟草根、茎、叶的总RNA,D(260)/D(280)为1.9~2.0,表明RNA纯度较好,可进行后续实验。如图 4A所示:NtPLR1在根、茎和叶均有表达,在叶中表达最高,根、茎表达水平较低。对不同逆境胁迫下NtPLR1的表达分析发现:紫外线胁迫下,随时间延长,NtPLR1基因在烟草叶片中的表达量表现出先升高后下降的趋势,并在紫外线处理4 h时达到最大值(图 4B)。氧化及氯化钠(100.0 mmol·L-1)浇灌处理时,随胁迫时间的增加,NtPLR1基因在烟草叶片中的表达持续升高,7 d时表达最高(图 4C)。在以上逆境胁迫下,NtPLR1表达呈现不同程度的上调,这表明NtPLR1与紫外线、氧化、氯化钠胁迫有应答反应。
-
烟草水培液添加外源PL后,分别于培养的第2天、第4天、第8天取样,分析NtPLR1基因的表达水平和PL,PN含量。定量PCR分析结果表明:NtPLR1表达随处理时间延长呈现先上升后下降的趋势,在第4天表达量达到最高,第2天、第4天、第8天的NtPLR1表达量分别是对照的2.20,2.85和1.50倍(图 5)。
VB6标准品高效液相色谱法(HPLC)检测结果如图 6A,峰型和区分度良好。对未处理的烟草叶片提取液分析发现,在PMP,PM,PLP,PL及PN的洗脱位置上均出现了相应的洗脱峰(图 6B),说明检测方法可行。据此,对PL处理组烟草叶片进行HPLC分析,结果表明:随时间延长,处理组烟草叶片中PL含量逐渐降低,PN含量增幅明显(图 7),同时,PMP,PM含量有小幅增长,表明烟草吸收外源PL后,主要将PL转化为PN。PL处理后NtPLR1的表达受到诱导,而在8 d时表达下降,结合PN,PMP和PM含量的逐渐增多可知,NtPLR1在烟草中催化PL形成PN。VB6各组分在烟草中动态转化,且各组分间存在反馈调节。
-
将重组质粒pET32a-NtPLR1转入BL21(DE3)Rosetta,分别在28 ℃和37 ℃条件下经异丙基硫代半乳糖苷(IPTG)诱导后,超声波破碎菌体,离心分离上清和沉淀。将上清和沉淀分别进行十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)检测,结果显示:上清中无目的条带,而沉淀中在约53 kDa处出现明显蛋白条带,因pET32a的组氨酸标签(his-tag)约为12 kDa,故蛋白条带的大小与预期相符。仅沉淀中出现目的条带,表明NtPLR1在大肠埃希菌中以包涵体形式存在(图 8)。
-
VB6在自然界中广泛地存在,主要以辅酶的形式参与生物体内多种物质代谢反应,是生物机体内很多重要酶系的辅酶[12]。植物体内,VB6参与淀粉、亚油酸等物质的合成[13],对于生长素、叶绿素以及乙烯的合成是不可或缺的[14-15]。近年来的研究还发现,VB6具有抗氧化作用,可猝灭超氧阴离子自由基及单线态氧[16];除此之外,在低温、渗透压、盐害、紫外及病菌等逆境中,VB6可以提高植株的抵抗力,发挥一定的抗逆作用[13, 17-20]。
VB6从头合成途径和补救合成途径普遍存在于植物和微生物中[21-24]。植物和微生物是VB6自养生物,动物自身无法从头合成VB6,只能从食物中获得VB6前体物质,通过补救合成途径满足机体对VB6的需求。VB6补救途径由多种酶参与,PLR是其中一种VB6补救合成酶,对于细胞进行正常生理活动具有重要意义。在研究拟南芥Atplr1时发现,Atplr1的VB6总水平下降,其中PL,PLP,PM和PMP水平显著下降,而PN和PNP无显著变化,推测在拟南芥内可能存在PLR的同工酶[6]。而HUANG等[25]的研究认为:烟草叶际PL—PN的转换可能受叶际微生物的影响较大。VB6对植物的生长发育、逆境适应及人和动物的营养具有重要意义,其从头合成途径已有较多的研究,而补救途径还有许多不明之处,有待深入研究。本研究从烟草中克隆得到烟草NtPLR1,并设置了不同的逆境胁迫条件对NtPLR1进行探究,结果表明:NtPLR1在叶中表达最高且受紫外线,氧化和氯化钠胁迫的诱导,推测NtPLR1参与烟草植株对紫外线、氧化和氯化钠胁迫的抗逆反应。外源添加PL后,NtPLR1表达量与对照相比显著上调,表明PL对NtPLR1有显著的诱导作用。此外,外源PL处理的前4 d NtPLR1的表达呈上升趋势,而在培养的第8天时NtPLR1的表达降低,相应的PL持续降低,而PN,PM和PMP等都有不同程度的升高,表明VB6各组分在烟草叶片中可相互转化并存在反馈调节。目前,本实验室正在进行NtPLR1重组蛋白的优化表达及体外酶活测定,以期为进一步探明烟草PLR基因功能及VB6补救合成过程奠定基础。
Cloning and expression analysis of the tobacco NtPLR1 gene
-
摘要: 维生素B6(VB6)在植物体内参与多种生化反应,对植物生长至关重要,吡哆醛还原酶(PLR)是VB6代谢转换的作用酶,催化吡哆醛(PL)生成吡哆醇(PN),对维持细胞内VB6的动态平衡发挥重要作用,而PLR在植物中鲜有报道。以拟南芥Arabidopsis thaliana吡哆醛还原酶氨基酸序列AtPLR1为模板,在公用数据库通过同源比对获得数条烟草Nicotiana tabacum NtPLR1基因的片段,结合互补脱氧核糖核酸(cDNA)的末端快速扩增-聚合酶链式反应(RACE-PCR)技术获得了烟草吡哆醛还原酶NtPLR1基因。该基因全长1 370 bp,编码369个氨基酸残基,预测其编码蛋白的分子量为41 kDa,理论等电点为9.42。氨基酸多序列比对结果表明:NtPLR1与其他物种的PLR1相似性较高。实时荧光定量PCR(qRT-PCR)分析结果表明:外源吡哆醛PL处理时,NtPLR1表达先升高后降低,在4 d达到顶峰。相应地,高效液相色谱分析结果表明:烟草叶片中PL含量随时间逐渐降低而吡哆醇PN含量逐渐升高,表明NtPLR1可像酵母PLR一样,催化PL形成PN。此外,定量分析结果表明:NtPLR1在烟草根、茎和叶片中均有表达,其中在叶片中表达显著高于其他部位(P < 0.05)。在紫外线、氧化和盐害胁迫下,NtPLR1的表达与对照相比均显著上调(P < 0.05),表明NtPLR1对这3种逆境有响应,可能参与烟草的抗逆过程。将NtPLR1连入原核表达载体pET32a,并进行诱导表达,成功表达出目的蛋白。报道的烟草NtPLR1基因功能为进一步探明植物PLR基因的功能和调控机制以及VB6的生物合成提供了重要参考。Abstract: Vitamin B6 (VB6), essential for plant growth and development and involved in more than 100 biological processes, utilizes pyridoxal reductase (PLR) as the key enzyme in the VB6 salvage pathway, thereby catalyzing pyridoxal (PL) to generate pyridoxine (PN). Since studies on PLR of plant VB6 are quite limited, PLR genes were cloned and characterized to improve understanding of VB6 biosynthesis in plants. Several NtPLR1 gene fragments were found in Nicotiana tabacum through a homologous blast with Arabidopsis AtPLR1. Full length was obtained using rapid amplification of cDNA ends (RACE). Real-time quantitative polymerase chain reaction (PCR) and high performance liquid chromatography (HPLC) analysis were conducted; NtPLR1 expression by ultraviolet, oxidation, exogenous PL, and NaCl treatments were compared to a control; and prokaryotic expression of NtPLR1 was accomplished. Results of RACE showed that full length cDNA of NtPLR1 was 1 370 bp, which encoded 369 amino acid residues with a protein molecular weight of about 41 kDa and a theoretical isoelectric point of 9.42. Real-time quantitative PCR analysis revealed that an exogenous PL treatment induced NtPLR1 expression with highest expression at 4 d. The HPLC analysis showed that PL content significantly decreased (P < 0.05); whereas, PN content significantly increased (P < 0.05) during an exogenous PL treatment. NtPLR1 was expressed in roots, stems, and leaves with leaves having the highest (P < 0.05) expression level. Also, ultraviolet, oxidation, and NaCl treatments, compared to a control, significantly induced (P < 0.05) NtPLR1 expression. Furthermore, prokaryotic expression of NtPLR1 in vector pET32a successfully revealed the recombinant protein at the expected size. This study reported the NtPLR1 gene of N. tabacum for the first time, finding that it catalyzed PL to form PN in tobacco as found in yeast, and it may be induced in response to ultraviolet, oxidation, and NaCl stress; thus, the NtPLR1 gene can be an important reference for further plant PLR gene functional characterization and regulation as well as VB6 biosynthesis.
-
Key words:
- botany /
- vitamin B6 /
- Nicotiana tabacum /
- NtPLR1 /
- gene cloning /
- expression analysis
-
表 1 NtPLR1基因克隆与表达分析所用引物信息
Table 1. Primers used in NtPLR1 gene cloning and expression analysis
用途 引物名称 引物序列(5'→3') 3'-RACE 3RACE-1 TGCAAATTATGCACCTCTGCAGGAACG 3RACE-2 TGCAGTTGGGGTGAGCAACTATGGACC 3RACE-3 TGCGCTCAGCCCAGGTACAATTTTCAT 目的片段扩增NtPLR1 NtPLR-F1 ATGGCTCTCTCACTCCCAGCTTCAAAATC NtPLR-R1 CTTTGTCTGAAATACGTTTTGGATC 原核表达 NtPLR-F2 GGATCCATGGCTCTCTCACTCCCAGCTTCAAAATC NtPLR-R2 GTCGACCTTTGTCTGAAATACGTTTTGGATC qRT-PCR NtPLR-F3 TGGCAAAAGGTAAAGATGGG NtPLR-R3 GTTGATGCCATTCTCCACCG 说明:下划线分别表示BamH1和Sal1。 -
[1] RAIL L C, NIKBIN M S. Vitamin B6 and immune competence[J]. Nutr Rev, 1993, 51(8): 217-225. [2] HUANG Shuohao, ZENG Haibin, ZHANG Jianyun, et al. Interconversions of different forms of vitamin B6 in tobacco plants[J]. Phytochemistry, 2011, 72(17): 2124-2129. [3] MORINO Y, SAKAMOTO Y. Enzymatic studies on pyridoxine metabolism (Ⅳ) a pyridoxine dehydrogenase from baker's yeast[J]. J Biochem, 1960, 48: 733-744. [4] HOLZER H, SCHNEIDER S. Purification and characterization of a TPN-dependent pyridoxol dehydrogenase from brewers yeast[J]. Biochim Biophys Acta, 1961, 48(2): 71-76. [5] OLLILAINEN V. HPLC analysis of vitamin B6 in foods[J]. Agric Food Sci Finland, 1999, 8(6): 515-619. [6] HERRERO S, GONZALEZ E, GILLIKIN J W, et al. Identification and characterization of a pyridoxal reductase involved in the vitamin B6 salvage pathway in Arabidopsis[J]. Plant Mol Biol, 2011, 76(1/2): 157-169. [7] 蒋守花, 张剑韵, 黄龙全.采用高效液相色谱技术分析茶树体内维生素B6[J].茶叶科学, 2010, 30(2): 79-82. JIANG Shouhua, ZHANG Jianyun, HUANG Longquan. Analysis of VB6 derivatives in tea plant with high performance liquid chromatography[J]. J Tea Sci, 2010, 30(2): 79-82. [8] 张剑韵, 黄龙全, 早川享志, 等.采用高效液相色谱技术分析生物体内维生素B6[J].高等学校化学学报, 2004, 25(4): 638-640. ZHANG Jianyun, HUANG Longquan, HAYAKAWA T, et al. Analysis of VB6 derivatives in biological samples with high performance liquid chromatography[J]. Chem J Chin Univ, 2004, 25(4): 638-640. [9] 曾海彬, 张剑韵, 黄龙全.采用高效液相色谱技术分析烟草体内的维生素B6化合物[J].广西植物, 2011, 31(5): 695-698. ZENG Haibin, ZHANG Jianyun, HUANG Longquan. Analysis of vitamin B6 vitamers in tobacco plants by high performance liquid chromatography[J]. Guihaia, 2011, 31(5): 695-698. [10] GUIRARD B M, SNELL E E. Physical and kinetic properties of a pyridoxal reductase purified from baker's yeast[J]. Biofactors, 1988, 1(2): 187-192. [11] NAKANO M, MORITA T, YAMAMOTO T, et al. Purification, molecular cloning, and catalytic activity of Schizosaccharomyces pombe pyridoxal reductase a possible additional family in the aldo-keto reductase superfamily[J]. J Biol Chem, 1999, 274(33): 23185-23190. [12] LYON J B, BAIN J A, WILLIAMS H L. The distribution of vitamin B6 in the tissues of two inbred strains of mice fed complete and vitamin B6-deficient rations[J]. J Biol Chem, 1962, 237(6): 1989-1991. [13] MOONEY S, HELLMANN H. Vitamin B6: killing two birds with one stone?[J]. Phytochemistry, 2010, 71(5/6): 495-501. [14] TAO Yi, FERRER J L, LJUNG K, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants[J]. Cell, 2008, 133(1): 164-176. [15] VAVILIN D V, VERMAAS W F J. Regulation of the tetrapyrrole biosynthetic pathway leading to heme and chlorophyll in plants and cyanobacteria[J]. Physiol Plantarum, 2002, 115(1): 9-24. [16] BILSKI P, LI M Y, EHRENSHAFT M, et al. Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants[J]. Photochem Photobiol, 2000, 71(2): 129-134. [17] SHI Huazhong, XIONG Liming, STEVENSON B, et al. The Arobidopsis salt overly sensitive 4 mutants uncover a critical role for vitamin B6 in plant salt tolerance[J]. Plant Cell, 2002, 14(3): 575-588. [18] CHEN Hao, XIONG Liming. Pyridoxine is required for post-embryonic root development and tolerance to osmotic and oxidative stresses[J]. Plant J, 2005, 44(3): 396-408. [19] ZHANG Yafen, JIN Xiaoyi, OUYANG Zhigang, et al. Vitamin B6 contributes to disease resistance against Pseudomonas syringae pv. tomato DC3000 and Botrytis cinerea in Arabidopsis thaliana[J]. J Plant Physiol, 2015, 175(1): 21-25. [20] 黄龙全, 张剑韵.植物维生素B6从头合成与代谢转换研究进展[J].西北植物学报, 2015, 35(10): 2124-2131. HUANG Longquan, ZHANG Jianyun. Review on the de novo synthesis and metabolic conversions of vitamin B6 in plants[J]. Acta Bot Boreal-Occident Sin, 2015, 35(10): 2124-2131. [21] YU Shunwu, LUO Lijun. Expression analysis of a novel pyridoxal kinase messenger RNA splice variant, PKL, in oil rape suffering abiotic stress and phytohormones[J]. Acta Biochim Biophys Sin, 2008, 40(12): 1005-1014. [22] SANG Yuying, BARBOSA J M, WU Hongzhuan, et al. Identification of a pyridoxine (pyridoxamine) 5'-phosphate oxidase from Arabidopsis thaliana[J]. FEBS Letters, 2007, 581(3): 344-348. [23] EHRENSHAFT M, BILSKI P, LI M Y, et al. A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis[J]. Proc Nat Acad Sci USA, 1999, 96(16): 9374-9378. [24] MITTENHUBER G. Phylogenetic analyses and comparative genomics of vitamin B6 (pyridoxine) and pyridoxal phosphate biosynthesis pathways[J]. J Mol Microbiol Biotechnol, 2001, 3(1): 1-20. [25] HUANG Shuohao, ZHANG Jianyun, TAO Zhen, et al. Enzymatic conversion from pyridoxal to pyridoxine caused by microorganisms within tobacco phyllosphere[J]. Plant Physiol Biochem, 2014, 85: 9-13. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2017.04.003







 下载:
下载: