-
扑草净(4,6-双异丙胺基-2-甲硫基-1,3,5-三嗪)是一种低毒、高选择的内吸性除草剂,因杀草谱广、药效长、除藻效果明显等优点被广泛应用于农业生产和水产养殖中[1],在水中溶解度低(25 ℃,48 mg·L−1),半衰期为1~3月,化学性质稳定,难降解,对生态环境的影响已经引起世界各国的关注[2]。2010年扑草净被农业部第1435号公告列入《兽药试行标准废止标准目录》[3],但许多地区仍将其作为水质改良剂大量用于水产养殖中,造成水环境污染,水产品扑草净残留检出甚至超标[4-5]。此外,扑草净是一种环境内分泌干扰物质,经食物链传递进入体内,可导致生物体内分泌系统、生殖器官、神经系统和免疫系统异常等,威胁生物体健康[6-8]。因此,解决扑草净对水环境的污染问题刻不容缓。植物修复是运用植物遏制、降解或提取环境中外源性污染物的技术,具有高效、环保、无公害、成本低的优点[9]。香根草Vetiveria zizanioides又名岩兰草,为禾本科Gramineae香根草属Vetiveria多年生C4草本植物,根系发达,生物量大,适应性广,是较理想的水土保持植物[10]。近年来,香根草在环境修复领域应用广泛,对重金属铁、镍、铬、锰等有较强的富集能力,可有效净化富营养化水体并吸收降解环境中的阿特拉津[11-13]。扑草净是一种三氮苯类除草剂,化学结构与阿特拉津相似。目前,尚未有香根草修复不同质量浓度扑草净污染水体的相关报道。因此,本研究利用温室水培试验,分析不同初始质量浓度扑草净污染下,水体扑草净质量浓度和香根草体内扑草净质量分数的动态变化,为应用香根草修复农药污染水体提供理论基础。
-
供试香根草购于江西红壤研究所,试验场地为西南林业大学格林温室,试验时间为2019年5−7月。选择苗龄及长势一致的香根草植株,以改良Hoagland营养液[14]为母液,稀释2倍后作为水培溶液。扑草净标准品购于济南仁诺化工有限公司。
-
设置香根草种植组(T)和未种植香根草组(N),在培养液中添加不同质量浓度(1.0、5.0、10.0、15.0 mg·L−1)扑草净,分别标记为T1、T5、T10、T15和N1、N5、N10、N15,以种植香根草未添加扑草净为对照(T0),于第0、5、10和15天分别取样,其中T组采集水体样品和植物样品,N组采集水体样品。
-
根据质量差法采集水样,用乙酸乙酯萃取。采用GC-MS(Thermo Fishsher,美国)测定水体中扑草净质量浓度。测试条件为:TG-5MS色谱柱(30 m×0.25 mm×0.25 µm),进样口温度250 ℃,离子传输线温度250 ℃,离子源温度250 ℃。升温程序为:初始温度40 ℃,保持1.0 min,35 ℃·min−1升至180 ℃,保持1.0 min,然后以20 ℃·min−1的速率升至280 ℃,保持1.5 min,不分流进样。进样量为1 µL;载气为氦气;扫描离子质荷比为184、241、58。
-
茎叶、根系分别称取(2.00±0.01) g,剪成2~3 mm碎片。用乙腈超声提取。采用GC-MS测定植物不同部位扑草净质量分数,仪器测定条件同水样。
-
转移系数FT=C1/C2,其中C1为茎叶扑草净质量分数(mg·kg−1),C2为根系扑草净质量分数(mg·kg−1)。
Ct=C0e−kt,其中Ct为t时间点水体扑草净质量浓度(mg·L−1),C0为水体扑草净初始质量浓度(mg·L−1),k为降解速率常数,t为施药后的天数(d)。
扑草净去除率R=[(C0−Ct)/C0]×100%。扑草净相对去除率R0=RT−RN,其中RT、RN分别为相同初始质量浓度及培养时间下,T组及N组扑草净去除率(%)。
采用WPS 2018整理数据,SPSS 19.0进行单因素方差分析,分析方法为Duncan比较法,采用R语言进行相关性分析,Oringin 2018进行绘图。
-
准确称取103 mg扑草净标准品,溶于100 mL乙腈中,得到1 000.0 mg·L−1扑草净标准储备液;然后用正己烷逐级稀释为标准溶液,测定色谱峰面积。结果表明:扑草净在0~20.0 mg·L−1范围内线性良好,线性回归方程为y=2.303x+1.727,相关系数为0.999。方法的检出限为0.001 mg·L−1。
取空白水样30 mL,植物样品各2.00 g,分别添加 0.5、1.0、1.5 mg·L−1标准溶液,每个加标水平3个重复。测定不同类型样品加标回收率。其中水样加标回收率为(88.76±4.62)%,根系为(98.23±4.04)%,茎叶为(94.38±4.03)%,相对标准偏差为(4.03~4.62)%,表明该方法符合实验要求。
-
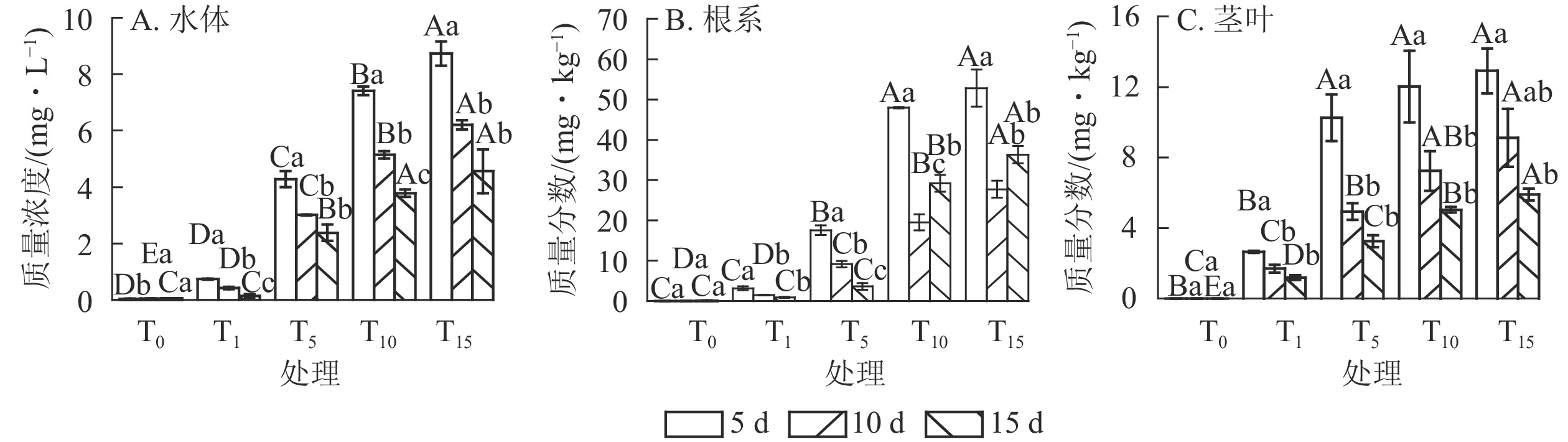
由图1A可以看出:随培养时间延长,T组水体扑草净质量浓度呈下降趋势。T1、T5处理,培养15 d水体扑草净较5 d分别降低了81.08%、44.39%,T10、T15处理分别降低了48.99%、47.77%。T1处理,培养15 d后水体扑草净质量浓度为0.14 mg·L−1,为培养10和5 d的33.33%和19.92%;T15处理,培养15 d后水体扑草净质量浓度为4.56 mg·L−1,为培养10和5 d的73.43%和52.23%。表明香根草对不同初始质量浓度扑草净污染水体具有一定修复效果。对不同培养时间下的水体扑草净质量浓度与初始质量浓度进行拟合,发现水体扑草净质量浓度与扑草净初始质量浓度呈线性关系,5、10、15 d拟合方程分别为:y5=0.50+0.60x、y10=0.31+0.43x、y15=0.21+0.32x,决定系数分别为0.961、0.965、0.950;培养时间越长,拟合方程斜率越小,说明扑草净初始质量浓度对水体扑草净质量浓度影响较大。

图 1 不同处理下水体扑草净质量浓度(A)、香根草根系(B)、茎叶(C)扑草净质量分数
Figure 1. Prometryn concentration of water (A), roots (B), shoots (C) of V. zizanioides under different treatments
由图1B可知:T组不同处理根系扑草净质量分数均为第5天最大,随培养时间延长,T1、T5处理下香根草根系扑草净质量分数下降,T10、T15处理下先下降后上升。不同处理根系扑草净质量分数培养10 d较5 d分别减少了52.37%、47.87%、59.30%、47.45%,15 d较5 d分别减少了71.29%、79.23%、39.14%、31.17%。推测5 d时其根部吸收扑草净达到最大值,随后向茎叶发生转移,根系继续吸收水培溶液中的扑草净,使得根系扑草净质量分数再次上升。对不同培养时间下的扑草净初始质量浓度与根系扑草净质量分数进行拟合,发现两者呈线性关系,5、10、15 d的拟合方程分别为:y5=0.52+3.84x 、 y10=−0.10+1.86x 、 y15=−2.41+2.65x,决定系数分别为0.952、0.988和0.929,说明不同培养时间下,扑草净初始质量浓度对香根草根系扑草净质量分数影响密切。
由图1C可知:T组不同处理下香根草茎叶中扑草净质量分数在培养5 d时达到峰值,随培养时间延长持续降低;不同处理茎叶中扑草净质量分数培养10 d较5 d分别降低了35.85%、51.80%、39.77%、29.43%,培养15 d时较5 d分别降低了55.09%、70.00%、58.07%、54.30%。对不同培养时间下的扑草净初始质量浓度与茎叶扑草净质量分数进行拟合,发现茎叶扑草净质量分数与扑草净初始质量浓度正相关,斜率随培养时间延长而减小。5、10、15 d的拟合方程分别为:y5=2.37+0.84 x 、y10=0.97+0.59x 、y15=0.70+0.38x ,决定系数分别为0.816、0.954和0.941,说明随着培养时间增加,香根草茎叶扑草净质量分数降低。
由表1可知:相同培养时间下,香根草转移系数均随扑草净初始质量浓度增加而降低。相比T1,培养5 d时T5、T10、T15香根草转移系数分别降低了30.95%、70.24%、71.43%,培养10 d时分别降低了54.46%、66.96%、70.54%,培养15 d时分别降低了32.06%、87.02%、87.79%。相同初始质量浓度处理下,香根草转移系数随培养时间延长呈波动趋势。T1、T5处理,香根草转移系数培养15 d较5 d分别增加55.95%、53.45%,T10、T15处理,香根草转移系数培养15 d较5 d分别降低32.00%、33.33%。表明香根草对水体扑草净的吸收、转移受初始质量浓度、培养时间影响。
表 1 不同处理下香根草转移系数
Table 1. Transfer coefficient of V. zizanioides under different treatments
t/d T1 T5 T10 T15 5 0.84±0.02 Ab 0.58±0.08 Bb 0.25±0.04 Cab 0.24±0.02 Cab 10 1.12±0.14 Aab 0.54±0.04 Bb 0.37±0.06 Ba 0.33±0.06 Ba 15 1.31±0.14 Aa 0.89±0.06 Ba 0.17±0.01 Cb 0.16±0.01 Cb 说明:大写字母表示不同处理间差异显著(P<0.05);小写字母表示不同培养时间下差异显著(P<0.05) -
采用一级反应动力学方程拟合不同培养时间下水体扑草净质量浓度的动态分布规律。水体添加不同质量浓度扑草净后,T组(图2A)和N组(图2B)水体扑草净的降解趋势均符合一级反应动力学方程,拟合度较高,培养时间与水体扑草净质量浓度均呈指数衰减趋势,最后逐渐趋于稳定。结合表2可知:不同初始质量浓度扑草净污染水体中,T组扑草净半衰期为7.42~13.82 d,N组为22.23~33.34 d,种植组较未种植组缩短了14.80~19.78 d;T组降解速率高于N组(P<0.05),表明种植香根草可加速水体中扑草净的降解,使降解半衰期提前。由表3可知:不同初始质量浓度扑草净污染的水体中,水体扑草净去除率T组高于N组(P<0.05);培养15 d,T组水体扑草净去除率为52.27%~85.88%,N组为29.16%~39.55%,去除率提高了22.52%~55.57%(P<0.05),表明香根草对不同初始质量浓度扑草净污染水体均有修复效果,1.0 mg·L−1初始质量浓度处理下,香根草对水体扑草净去除率最高。
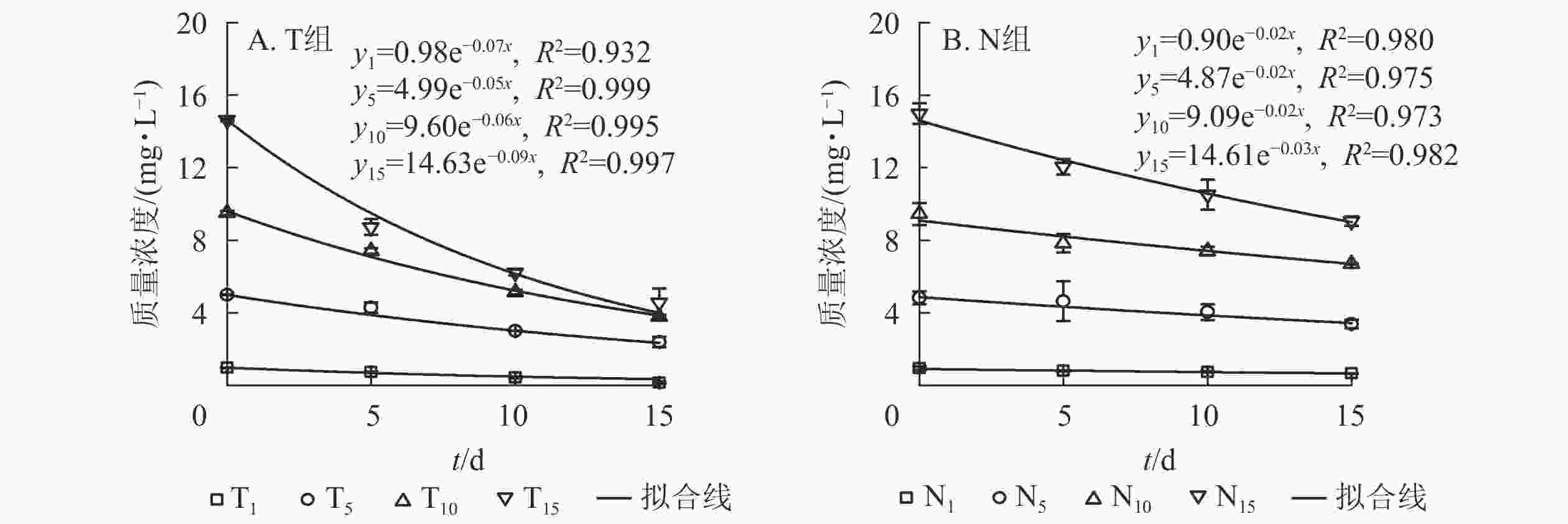
图 2 不同处理下水体扑草净质量浓度与培养时间的关系
Figure 2. Relationship between prometryn concentration and cultivation time under different treatments
表 2 不同处理下水体扑草净降解速率和半衰期
Table 2. Degradation rate and half-life of prometryn in water under different treatments
初始质量浓度/
(mg·L−1)T组 N组 降解速率/
d−1半衰期/
d降解速率/
d−1半衰期/
d1.0 0.07 b 9.61 c 0.02 a 29.39 b 5.0 0.05 c 13.82 a 0.02 a 33.34 a 10.0 0.06 bc 10.88 b 0.02 a 29.89 b 15.0 0.09 a 7.42 d 0.03 a 22.23 c 说明:不同小写字母表示差异显著(P<0.05) 表 3 不同处理下水体扑草净去除率
Table 3. Prometryn removal rate in water under different treatments
t/d 初始质量浓度/
(mg·L−1)去除率/% T组 N组 相对去除率 5 1.0 23.28±2.26 g 15.72±4.63 c 3.90±1.41 d 5.0 19.93±0.36 g 16.88±0.62 c 3.05±0.08 d 10.0 22.05±1.63 g 17.11±3.10 c 4.94±1.58 d 15.0 40.38±2.95 e 19.57±1.64 c 20.82±4.59 c 10 1.0 56.44±4.68 c 21.68±4.10 c 40.72±8.53 b 5.0 39.64±0.23 e 21.11±3.12 c 22.76±0.38 c 10.0 45.90±1.37 de 21.77±1.46 c 28.80±1.98 bc 15.0 57.56±1.14 c 29.80±3.22 b 37.99±2.31 b 15 1.0 85.88±6.58 a 30.30±1.03 b 55.57±5.66 a 5.0 52.27±4.06 cd 29.75±2.87 b 22.52±4.48 c 10.0 60.25±1.34 bc 29.16±0.15 b 31.09±1.20 bc 15.0 68.82±5.27 b 39.55±0.99 a 29.27±5.65 bc 说明:不同小写字母表示差异显著(P<0.05) -
对扑草净初始质量浓度与香根草不同部位扑草净质量分数及水体扑草净质量浓度、转移系数、去除率作相关性分析。由表4可知:扑草净初始质量浓度与香根草转移系数呈极显著负相关(P<0.01),与香根草茎叶、根系扑草净质量分数呈极显著正相关(P<0.01),与相对去除率相关性不显著(P>0.05),相对去除率与培养时间呈极显著正相关(P<0.01),与转移系数相关性不显著(P>0.05);转移系数与水体扑草净质量浓度、香根草茎叶、根系扑草净质量分数均呈极显著负相关(P<0.01)。
表 4 扑草净初始质量浓度与香根草不同部位扑草净质量分数、相对去除率的相关性
Table 4. Correlation between initial concentration and the concentration of prometryn in V. zizanioides and relative removal rate
项目 初始质量浓度 培养时间 相对去除率 转移系数 水体质量浓度 茎叶质量分数 根系质量分数 初始质量浓度 1 培养时间 0.000 1 相对去除率 −0.033 0.662** 1 转移系数 −0.844** 0.163 0.328 1 水体质量浓度 0.852** −0.396* −0.349* −0.798** 1 茎叶质量分数 0.659** −0.564** −0.441** −0.610** 0.875** 1 根系质量分数 0.850** −0.298 −0.286 −0.803** 0.916** 0.793** 1 说明:*表示显著相关(P<0.05),**表示极显著相关(P<0.01) -
水体农药污染修复技术中,植物修复因无二次污染、修复彻底等优点成为研究的热点[14],核心要素是植物的选择,因此,明确香根草对不同质量浓度扑草净的净化潜力尤为关键。研究发现:种植灯心草Juncus effusus和美人蕉Canna indica可显著提高农药的去除效率。本研究发现:培养15 d时,香根草种植组水体扑草净去除率较未种植组提高了22.52%~55.57%,表明香根草对扑草净污染水体有修复效果。与LÜ等[15]和董静等[16]研究一致。但不同植物对农药的吸收和降解效果存在差异。研究发现:芦苇Phragmites australis对特丁津的去除率高于宽叶香蒲Typha latifolia[17],蓉草Leersia oryzoides对莠去津、二嗪农的去除率高于宽叶香蒲[18]。NI等[19]研究表明:培养30 d时,铜钱草Hydrocotyle vulgaris对0.5 mg·L−1扑草净去除率较未种植植物提高了37%,就扑草净的去除效果而言,香根草优于铜钱草,可能与初始质量浓度、植物种类、生物量和根际微环境差异等多个因素有关。
自然环境中,水体中农药的去除或降解受多种因素影响,如水解、水/有机物分配、微生物降解等[20]。植物可提高降解效率,缩短农药半衰期[21]。本研究发现:不同初始质量浓度扑草净处理下,相比未种植组,香根草种植组水体扑草净半衰期显著缩短;但T15半衰期较其他处理长,可能是该质量浓度已经胁迫到香根草的正常生理活动,其修复效果受到影响,但与N15相比,仍提高了修复效率。
研究植物对有机污染物的吸收、转移特征,对明确有机污染物的环境行为、生态风险评价及植物修复等都具有重要的意义[22]。本研究中香根草体内扑草净质量分数主要与扑草净初始质量浓度有关,这与王庆海等[23]对黄菖蒲Iris pseudacorus的研究结果基本一致。本研究中各处理香根草根系扑草净质量分数培养第5天达到峰值,继续培养,高质量浓度(T10、T15)下先下降后上升,原因可能是培养5 d时,香根草根部吸收扑草净量达到饱和,随后向地上部转移,根系继续吸收水体中的扑草净。香根草茎叶扑草净质量分数与扑草净初始质量浓度呈正相关,与培养时间呈负相关。这与JIN等[24]对绿藻研究结果相似。SUN等[25]也发现香根草根系对扑草净的吸收并不呈线性关系,而是呈先上升后波动下降的趋势,与本研究一致。
转移系数可作为评价植物从根系向茎叶转移扑草净能力的指标[26]。不同培养时间下,香根草转移系数均随扑草净初始质量浓度增加而降低。低质量浓度(1.0 mg·L−1)时,转移系数大于1。这表明低质量浓度胁迫时,通过从地下部分向地上部分转移扑草净,植物根系与地上部一起协同吸收降解扑草净。高质量浓度(10 mg·L−1)时,转移系数先升高后降低,可能是随着胁迫质量浓度升高,植物地下部分向地上部分的物质传输能力降低,水体扑草净的去除以根系吸收降解扑草净为主。与何发林等[27]的结论一致。低质量浓度农药胁迫下,香根草根系活力提高,代谢速率升高,而高质量浓度农药则会对植物产生毒害作用,植物生长受到抑制甚至死亡。植物将大部分污染物滞留或固定在根部,阻止或减少其向地上部分运输,被认为是植物减轻对地上部的毒害,增强植物耐性的重要机制之一[28]。这也是高质量浓度扑草净胁迫下,香根草转移系数降低的原因,与宋清梅等[29]研究结果一致。
相关性分析表明:扑草净初始质量浓度与香根草转移系数呈显著负相关(P<0.05),去除率与培养时间呈极显著正相关(P<0.01),表明随扑草净初始质量浓度增加,转移系数降低;不同初始质量浓度扑草净胁迫下,香根草相对去除率变化不大,但随培养时间延长而增大。说明扑草净污染水体中香根草存在根际效应[30],即植物对污染物的去除效率与其根部生物量密切相关。香根草适应性广,根系发达,生物量大[31],是植物修复扑草净污染水体的理想选择。
Remediation potential of Vetiveria zizanioides on the water polluted with prometryn
-
摘要:
目的 探究香根草Vetiveria zizanioides对扑草净污染水体的修复潜力。 方法 采用温室水培模拟实验的方法,研究香根草对水体不同初始质量浓度(1.0、5.0、10.0、15.0 mg·L−1)扑草净的吸收和去除特征。 结果 相同培养时间下,随水体扑草净初始质量浓度增加,香根草茎叶和根系扑草净质量分数显著增加(P<0.05),在第5天达到最大值,之后呈波动下降趋势;随培养时间延长,水体扑草净质量浓度显著降低(P<0.05)。与未种植香根草相比,香根草种植组水体扑草净降解半衰期缩短了14.80~19.78 d,去除率提高了22.52%~55.57%。相关性分析表明:水体扑草净质量浓度与香根草转移系数呈极显著负相关(P<0.01),与相对去除率相关性不显著(P>0.05),与培养时间呈极显著正相关(P<0.01)。 结论 种植香根草可提高水体扑草净降解速率和去除率,香根草可作为扑草净污染水体修复的先锋植物。图2表4参31 Abstract:Objective This study is aimed to explore the remediation potential of Vetiveria zizanioides on prometryn polluted water. Method The characteristics of absorption and removal of prometryn by V. zizanioides with different initial concentrations (1.0, 5.0, 10.0, 15.0 mg·L−1)were studied by hydroponic simulation experiment in greenhouse. Result With the same cultivation time, the increase of initial prometryn concentration brought about a significant increase of prometryn concentration (P<0.05) in the shoots and roots of V. zizanioides, which reached the maximum on the 5th day, and then demonstrated a fluctuating decline. The extension of cultivation time led to a significant decrease in prometryn concentration in water (P< 0.05). Compared with the control without V. zizanioides, the degradation half-life of prometryn in the treatment group with V. zizanioides was shortened by 14.80−19.78 days, and the removal rate was increased by 22.52%−55.57%. The concentration of prometryn in water had a significant negative correlation with the transfer coefficient of V. zizanioides (P<0.01), no significant correlation with relative removal rate (P>0.05), but a significant positive correlation with the cultivation time (P<0.01). Conclusion The planting of V. zizanioides can promote the degradation rate and removal rate of prometryn, therefore, V. zizanioides can be used as a pioneer plant in the remediation of prometryn polluted water. [Ch, 2 fig. 4 tab. 31 ref.] -
Key words:
- Vetiveria zizanioides /
- prometryn /
- absorption and removal /
- rhizosphere effect /
- phytoremediation
-
表 1 不同处理下香根草转移系数
Table 1. Transfer coefficient of V. zizanioides under different treatments
t/d T1 T5 T10 T15 5 0.84±0.02 Ab 0.58±0.08 Bb 0.25±0.04 Cab 0.24±0.02 Cab 10 1.12±0.14 Aab 0.54±0.04 Bb 0.37±0.06 Ba 0.33±0.06 Ba 15 1.31±0.14 Aa 0.89±0.06 Ba 0.17±0.01 Cb 0.16±0.01 Cb 说明:大写字母表示不同处理间差异显著(P<0.05);小写字母表示不同培养时间下差异显著(P<0.05) 表 2 不同处理下水体扑草净降解速率和半衰期
Table 2. Degradation rate and half-life of prometryn in water under different treatments
初始质量浓度/
(mg·L−1)T组 N组 降解速率/
d−1半衰期/
d降解速率/
d−1半衰期/
d1.0 0.07 b 9.61 c 0.02 a 29.39 b 5.0 0.05 c 13.82 a 0.02 a 33.34 a 10.0 0.06 bc 10.88 b 0.02 a 29.89 b 15.0 0.09 a 7.42 d 0.03 a 22.23 c 说明:不同小写字母表示差异显著(P<0.05) 表 3 不同处理下水体扑草净去除率
Table 3. Prometryn removal rate in water under different treatments
t/d 初始质量浓度/
(mg·L−1)去除率/% T组 N组 相对去除率 5 1.0 23.28±2.26 g 15.72±4.63 c 3.90±1.41 d 5.0 19.93±0.36 g 16.88±0.62 c 3.05±0.08 d 10.0 22.05±1.63 g 17.11±3.10 c 4.94±1.58 d 15.0 40.38±2.95 e 19.57±1.64 c 20.82±4.59 c 10 1.0 56.44±4.68 c 21.68±4.10 c 40.72±8.53 b 5.0 39.64±0.23 e 21.11±3.12 c 22.76±0.38 c 10.0 45.90±1.37 de 21.77±1.46 c 28.80±1.98 bc 15.0 57.56±1.14 c 29.80±3.22 b 37.99±2.31 b 15 1.0 85.88±6.58 a 30.30±1.03 b 55.57±5.66 a 5.0 52.27±4.06 cd 29.75±2.87 b 22.52±4.48 c 10.0 60.25±1.34 bc 29.16±0.15 b 31.09±1.20 bc 15.0 68.82±5.27 b 39.55±0.99 a 29.27±5.65 bc 说明:不同小写字母表示差异显著(P<0.05) 表 4 扑草净初始质量浓度与香根草不同部位扑草净质量分数、相对去除率的相关性
Table 4. Correlation between initial concentration and the concentration of prometryn in V. zizanioides and relative removal rate
项目 初始质量浓度 培养时间 相对去除率 转移系数 水体质量浓度 茎叶质量分数 根系质量分数 初始质量浓度 1 培养时间 0.000 1 相对去除率 −0.033 0.662** 1 转移系数 −0.844** 0.163 0.328 1 水体质量浓度 0.852** −0.396* −0.349* −0.798** 1 茎叶质量分数 0.659** −0.564** −0.441** −0.610** 0.875** 1 根系质量分数 0.850** −0.298 −0.286 −0.803** 0.916** 0.793** 1 说明:*表示显著相关(P<0.05),**表示极显著相关(P<0.01) -
[1] GADELHA J R, ROCHA A C, CAMACHO C, et al. Persistent and emerging pollutants assessment on aquaculture oysters (Crassostrea gigas) from NW portuguese coast (ria de aveiro) [J]. Sci Total Environ, 2019, 666: 731 − 742. [2] 陈溪, 刘梦遥, 曲世超, 等. 海产品、底泥、海水中扑草净药物残留量的液相色谱-串联质谱检测[J]. 化学通报, 2013, 76(2): 183 − 186. CHEN Xi, LIU Mengyao, QU Shichao, et al. Determination of prometryne residues in seafood, sediment, seawater by liquid chromatography tandem mass spectrometry [J]. Chemistry, 2013, 76(2): 183 − 186. [3] 中华人民共和国农业农村部. 中华人民共和国农业部公告1435号[EB/OL]. (2010-08-23)[2020-9-15]. http://www.moa.gov.cn/gk/tzgg_1/gg/201008/t20100823_1622639.htm. [4] SAKA M, TADA N, KAMATA Y, et al. Chronic toxicity of 1,3,5-triazine herbicides in the postembryonic development of the western clawed frog Silurana tropicalis [J]. Ecotox Environ Safe, 2018, 147: 373 − 381. [5] TIAN Yaya, LIU Minxuan, SANG Yaxin, et al. Degradation of prometryn in ruditapes philippinarum using ozonation: influencing factors, degradation mechanism, pathway and toxicity assessment [J]. Chemosphere, 2020, 248: 126018. doi: 10.1016/j.chemosphere.2020.126018. [6] 桂英爱, 葛祥武, 孙程鹏, 等. 扑草净在环境和生物体内的降解代谢、毒性及安全性评价研究进展[J]. 大连海洋大学学报, 2019, 34(6): 846 − 852. GUI Ying’ai, GE Xiangwu, SUN Chengpeng, et al. Research progress: degradation, metabolism, toxicity and safety evaluation of prometryne in environment and organisms [J]. J Dalian Ocean Univ, 2019, 34(6): 846 − 852. [7] NEVADO J J B, CABANILLAS C G, LLERENA M J V, et al. Sensitive SPE GC-MS-SIM screening of endocrine-disrupting herbicides and related degradation products in natural surface waters and robustness study [J]. Microchem J, 2007, 87(1): 62 − 71. [8] RHIND S M, KYLE C E, RUFFIE H, et al. Short- and long-term temporal changes in soil concentrations of selected endocrine disrupting compounds (EDCs) following single or multiple applications of sewage sludge to pastures [J]. Environ Pollut, 2013, 181: 262 − 270. [9] 徐炜杰, 郭佳, 赵敏, 等. 重金属污染土壤植物根系分泌物研究进展[J]. 浙江农林大学学报, 2017, 34(6): 1137 − 1148. XU Weijie, GUO Jia, ZHAO Min, et al. Research progress of soil plant root exudates in heavy metal contaminated soil [J]. J Zhejiang A&F Univ, 2017, 34(6): 1137 − 1148. [10] 刘枭宏, 李铁, 谌芸, 等. 香根草植物篱带宽对紫色土坡地产流产沙的影响[J]. 水土保持学报, 2019, 33(4): 93 − 101. LIU Xiaohong, LI Tie, CHEN Yun, et al. Effects of Vetiveria zizanioides L. hedgerow width on runoff and sediment yield on purple soil slope [J]. J Soil Water Conserv, 2019, 33(4): 93 − 101. [11] BANERJEE R, GOSWAMI P, PATHAK K, et al. Vetiver grass: an environment clean-up tool for heavy metal contaminated iron ore mine-soil [J]. Ecol Eng, 2016, 90: 25 − 34. [12] MATHEW M, ROSARY S C, SEBASTIAN M, et al. Effectiveness of vetiver system for the treatment of wastewater from an institutional kitchen [J]. Proc Technol, 2016, 24: 203 − 209. [13] MARCACCI S, RAVETON M, RAVANEL P, et al. Conjugation of atrazine in vetiver (Chrysopogon zizanioides Nash) grown in hydroponics [J]. Environ Exp Bot, 2006, 56(2): 205 − 215. [14] ALENCAR B T B, RIBEIRO V H V, CABRAL C M, et al. Use of macrophytes to reduce the contamination of water resources by pesticides [J]. Ecol Indic, 2020, 109: 105785. doi: 10.1016/j.ecolind.2019.105785. [15] LÜ Tao, CARVALHO P N, ZHANG Liang, et al. Functionality of microbial communities in constructed wetlands used for pesticide remediation: influence of system design and sampling strategy [J]. Water Res, 2017, 110: 241 − 251. [16] 董静, 王立, 马放, 等. AMF对美人蕉修复水体除草剂污染的影响[J]. 哈尔滨工业大学学报, 2017, 49(2): 37 − 43. DONG Jing, WANG Li, MA Fang, et al. Effects of arbuscular mycorrhizal fungi inoculation on the phytoremediation of herbicide by Canna indica L. var flava Roxb. in water [J]. J Harbin Ins Technol, 2017, 49(2): 37 − 43. [17] GIKAS G D, PÉREZ-VILLANUEVA M, TSIORAS M, et al. Low-cost approaches for the removal of terbuthylazine from agricultural wastewater: constructed wetlands and biopurification system [J]. Chem Eng J, 2018, 335(1): 647 − 656. [18] MOORE M T, TYLER H L, LOCKE M A. Aqueous pesticide mitigation efficiency of Typha latifolia (L.), Leersia oryzoides (L.) Sw., and Sparganium americanum Nutt. [J]. Chemosphere, 2013, 92(10): 1307 − 1313. [19] NI J, SUN Shixian, ZHENG Yi, et al. Removal of prometryn from hydroponic media using marsh pennywort (Hydrocotyle vulgaris L.) [J]. Int J Phytoremediat, 2018, 20(9): 909 − 913. [20] CHULUUN B, IAMCHATURAPATR J, RHEE J S. Phytoremediation of organophosphorus and organochlorine pesticides by Acorus gramineus [J]. Environ Eng Res, 2009, 14(4): 226 − 236. [21] 贾继维, 张坤, 李鑫圆, 等. 腐植酸对香根草吸收和去除扑草净的影响[J]. 西南林业大学学报, 2019, 39(2): 121 − 126. JIA Jiwei, ZHANG Kun, LI Xinyuan, et al. The effect of humic acid on the uptake and removal of prometryn by vetiver grass [J]. J Southwest For Univ, 2019, 39(2): 121 − 126. [22] 莫凌, 张云, 林彰文, 等. 植物对卤代有机污染物吸收、迁移和代谢的研究进展[J]. 生态环境学报, 2015, 24(9): 1582 − 1590. MO Ling, ZHANG Yun, LIN Zhangwen, et al. Absorption, translocation and metabolism of halogenated organic pollutants (HOPs) in plants: a review [J]. Ecol Environ Sci, 2015, 24(9): 1582 − 1590. [23] 王庆海, 夏凡, 李翠, 等. 黄菖蒲对水中阿特拉津污染的去除贡献研究[J]. 农业环境科学学报, 2020, 39(11): 2613 − 2620. WANG Qinghai, XIA Fan, LI Cui, et al. Contribution of Iris pseudacorus to atrazine dissipation in water: effects of initial atrazine concentrations [J]. J Agro-Environ Sci, 2020, 39(11): 2613 − 2620. [24] JIN Zhenpeng, LUO Kai, ZHANG Shuang, et al. Bioaccumulation and catabolism of prometryne in green algae [J]. Chemosphere, 2012, 87(3): 278 − 284. [25] SUN Shixian, LI Yongmei, ZHENG Yi, et al. Uptake of 2,4-bis(isopropylamino)-6-methylthio-s-triazine by vetiver grass (Chrysopogon zizanioides L.) from hydroponic media [J]. Bullet Environ Contam Toxicol, 2016, 96(4): 550 − 555. [26] BAUMANN J, ANFT T, DOUGHTY K J, et al. Exposure to pesticide residues during manual removal of bolting sugar beets: determination of transfer coefficients for worker risk assessment [J]. J Consum Prot Food Safety, 2019, 14: 283 − 286. [27] 何发林, 曹莹莹, 李冠群, 等. 氯虫苯甲酰胺拌种对玉米种子活力及幼苗生长的影响[J]. 中国农学通报, 2019, 35(15): 151 − 158. HE Falin, CAO Yingying, LI Guanqun, et al. Effects on seed activity and seedling growth of maize by seed dressing with chlorantraniliprole [J]. Chin Agric Sci Bull, 2019, 35(15): 151 − 158. [28] 王学华, 戴力. 作物根系镉滞留作用及其生理生化机制[J]. 中国农业科学, 2016, 49(22): 4323 − 4341. WANG Xuehua, DAI Li. Immobilization effect and its physiology and biochemical mechanism of the cadmium in crop roots [J]. Sci Agric Sin, 2016, 49(22): 4323 − 4341. [29] 宋清梅, 蔡信德, 吴颖欣, 等. 香根草对污染土壤水溶态重金属组分胁迫响应研究[J]. 农业环境科学学报, 2019, 38(12): 2715 − 2722. SONG Qingmei, CAI Xinde, WU Yingxin, et al. Response of Vetiveria zizanioides to the stress of water-soluble components of heavy metals in contaminated soil [J]. J Agro-Environ Sci, 2019, 38(12): 2715 − 2722. [30] 潘声旺, 雷志华, 吴云霄, 等. 苏丹草根分泌物在有机氯农药降解过程中的作用[J]. 中国环境科学, 2017, 37(8): 3072 − 3079. PAN Shengwang, LEI Zhihua, WU Yunxiao, et al. Effect of exudates from Sorghum sudanense grass roots on degradation of organochlorine pesticides in soils [J]. China Environ Sci, 2017, 37(8): 3072 − 3079. [31] 李珍玉, 王丽锋, 肖宏彬, 等. 香根草根系在公路边坡土体中的分布特征[J]. 应用基础与工程科学学报, 2017, 25(1): 102 − 112. LI Zhenyu, WANG Lifeng, XIAO Hongbin, et al. Distribution characteristics of vetiver’s roots in highway slope [J]. J Basic Sci Eng, 2017, 25(1): 102 − 112. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20200595






 下载:
下载: