-
七叶一枝花Paris polyphylla是百合科Liliaceae重楼属Paris多年生草本植物,为中国特有的名贵药材重楼的基源植物之一,以根茎入药,具有清热解毒、消肿止痛、凉肝定惊等功效,民间多用于治疗无名肿毒和毒蛇咬伤,以及流行性腮腺炎、扁桃体炎、乳腺炎、咽喉肿痛、跌打伤痛、惊风抽搐等症[1]。现代药理研究表明,重楼还具有抗癌止血、祛痰抑菌、止咳平喘、镇静镇痛、抗早孕杀灭精子和抗细胞毒等功效,是中国云南白药、宫血宁、百宝丹、热毒清、季德盛蛇药、抗病毒颗粒、总皂苷片等许多著名中成药的主要原料,具有广阔的开发应用前景[2-3]。据《浙江植物志》和《浙江省药用资源名录》记载,七叶一枝花作为浙江的珍贵野生药材资源,在浙江各地均有分布[4-5]。但长期以来,掠夺式的过度采挖以及生态环境的恶化,严重危及七叶一枝花的自然生长与繁衍,其野生种群的自我更新能力也受到极大破坏。目前浙江多处七叶一枝花资源已非常稀少,难以寻见,濒于灭绝的境地[6-10]。因此,七叶一枝花的研究工作逐步为人们重视,主要包括基于16S rDNA和28S rDNA基因间隔序列(ITS序列)亲缘关系、药理活性、化学成分分析、快繁及人工栽培技术等研究[11-14]。ISSR(Inter-simple sequence repeat,简单重复序列间扩增)是由Zietkiewicz等[15]于1994年创建的一种基于聚合酶链式反应(PCR)的新型分子标记,它以能快速高效灵敏地检测出基因组DNA的多态性,并具有良好的重复性,操作简便、成本低、DNA用量小、安全性较高等特点,而被广泛应用于品种鉴定、遗传关系及遗传多样性、基因标记、植物基因作图、指纹图谱的建立等多方面的研究[16]。近年来,ISSR技术迅速在药用植物的种源鉴定、种质遗传多样性分析、亲缘关系分析、药用植物辅助育种等研究中得到应用,取得了较好的效果,也越来越受到研究者的重视[17-18]。ISSR是基于PCR反应的一种分子标记,影响反应的因素较多,不同物种所需的各种技术参数也存在一定差异。为了在七叶一枝花研究中获得稳定可靠、重复性好的分析结果,本研究利用单因素试验初筛和正交设计实验筛选结合的方法,对影响PCR反应的dNTPs,TaqDNA聚合酶,引物,模板DNA和镁离子(Mg2+)浓度及引物退火温度等技术参数进行优化筛选,以建立适合七叶一枝花ISSR-PCR的最佳反应体系和反应条件,为后续的种源鉴定及遗传多样性评价奠定基础。
HTML
-
试验材料为采于浙江省临安市东天目山海拔867 m野生七叶一枝花新鲜叶片,洗净,保存于-80 ℃超低温冰箱中。试验所用ISSR引物由生工生物工程(上海)有限公司合成,PCR反应试剂三磷酸碱基脱氧核苷酸(dNTPs)和Taq DNA聚合酶均购自宝生物工程(大连)有限公司,利用Eppendorf AG PCR仪进行反应扩增。
-
采用改良的4×十六烷基三甲基溴化铵(CTAB)法[19]提取七叶一枝花的总DNA,然后用10.0 g·kg-1的琼脂糖凝胶电泳检测其质量,用ND-1000分光光度计对DNA的浓度进行测定。根据测定结果,用灭菌去离子水将DNA浓度稀释至100 mg·L-1,并保存至-20 ℃以备用。
-
采用上海生工合成的ISSR引物,通过筛选,选择引物ISSR2(5′-GACAGACAGACAGACA-3′)用于七叶一枝花反应系统的优化。
-
采用单因素设计方法,参考北重楼Paris vertcillata,黑籽重楼Paris thibetica等[20-22] ISSR-PCR反应条件,对七叶一枝花ISSR反应体系中的TaqDNA聚合酶,模板DNA,dNTPs,引物,Mg2+,退火温度等6种主要因子设置不同浓度水平进行分析(25.0 μL反应体系中各成分优化设计方案见表 1)。
Taq酶/(×16.67nkat) DNA模板/ng dNTPs/ (mmol • L-1) 引物/(μmol.L-1) Mg2+/(mmol.L-1) 退火温度/℃ 0.25 10 0.05 0.2 1.0 46 0.50 20 0.10 0.3 1.5 48 0.75 30 0.15 0.4 2.0 50 1.00 40 0.20 0.5 2.5 52 1.25 50 0.25 0.6 3.0 54 1.50 60 0.30 0.7 3.5 56 Table 1. Single factors design of ISSR-PCR for Paria polyphylla
-
在单因素试验的基础上,采用正交设计方法,选择各因素之间的优化组合。
-
PCR扩增程序为:95 ℃预变性5 min;94 ℃变性40 s;52 ℃退火45 s;72 ℃延伸90 s;40次循环;72 ℃延伸7 min;4 ℃保存。PCR反应产物检测采用15 g·kg-1的Tris-乙酸(TAE)琼脂糖凝胶,使用杭州纽龙生物科技有限公司生产DNA Marker Ⅱ Plus,用0.5 μg·L-1溴化乙锭(EB)染色,以1×Tris-乙酸(TAE)为电泳缓冲液在150 V电压下电泳20~25 min,于Alphalmager HP凝胶成像系统进行拍照分析。
2.1. 基因组DNA提取与测定
2.2. ISSR-PCR反应体系的优化
2.2.1. 单因素试验设计
2.2.2. 正交设计试验优化
2.3. PCR扩增与扩增产物的检测
-
用改良CTAB法所提的七叶一枝花DNA的吸光度D(260/280)值为1.8~2.0,表明DNA纯度较好,电泳检测发现条带完整,无RNA污染,说明该法适合七叶一枝花DNA的提取,所提DNA质量较好,适用于ISSR-PCR扩增。
-
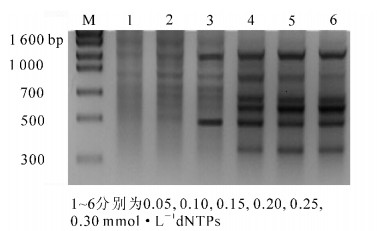
dNTPs是PCR反应的原料,不同dNTPs浓度对七叶一枝花PCR扩增结果会产生影响(图 1)。由图 1可看出:当dNTPs浓度为0.05~0.10 mmol·L-1时扩增出的条带较为模糊,这主要是因dNTPs浓度过低引发PCR产物合成率下降所致;随着dNTPs浓度的增加,扩增条带逐渐明亮清晰,dNTPs浓度在0.20~0.25 mmol·L-1时效果最佳;当dNTPs浓度增加到0.3 mmol·L-1时,条带趋于消失。过高不但造成浪费,而且会降低Mg2+的有效浓度和产生错误掺入从而会产生非特异性扩增。
-
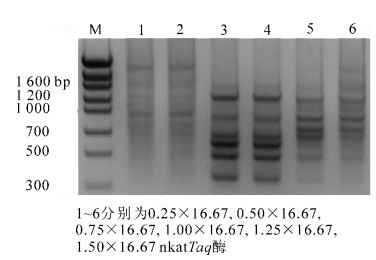
不同浓度的TaqDNA聚合酶对PCR扩增效果产生了明显影响(图 2),Taq酶在0.25 × 16.67 nkat(0.25 U)和0.50 × 16.67 nkat(0.5 U)时虽有扩增,但条带非常微弱,难以辨识,说明酶量过少影响了扩增效率;当其浓度在0.75×16.67 nkat(0.75 U)和1.0×16.67 nkat (1.0 U)时扩增效果较好,条带清晰可辨;而当酶浓度在1.25×16.67 nkat(1.25 U)以上时,条带亮度和清晰度下降,且有错误扩增出现。
-
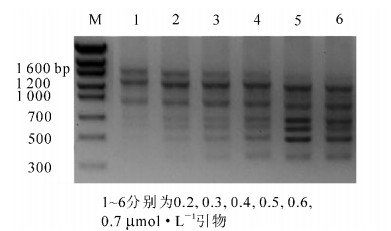
不同引物浓度对七叶一枝花PCR扩增的结果如图 3所示。由图 3可以看出:引物浓度对ISSR-PCR有很大影响,引物浓度过低时仅能扩增出3条大片段的条带,随着引物浓度的增加,小于1 000 bp的条带逐渐显现,在浓度为0.6 μmol·L-1左右时,条带清晰可辨,扩增效果最好。而浓度为0.7 μmol·L-1时,扩增条带重又变得模糊不清,引物浓度过高还会增加引物之间形成二聚体的概率。
-
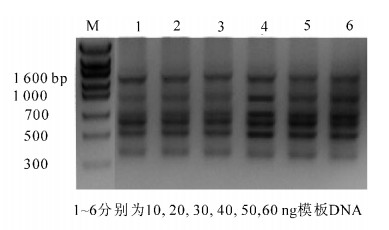
在25.0 μL的ISSR-PCR反应体系中,七叶一枝花模板DNA在10,20,30,40,50,60 ng时的扩增结果见图 4。模板DNA在10~60 ng范围内均能扩增出数量相同但清晰度不同的条带,说明模板用量对PCR扩增的条带数影响相对较小,但用量过低或过高都会影响条带的清晰度,模板用量过高,还会出现条带拖尾现,模板用量为40~60 ng较好。本研究用量在40 ng时扩增出的条带最为清晰可辨。
-
不同镁离子(Mg2+)浓度对七叶一枝花PCR扩增的结果如图 5所示。Mg2+浓度对PCR扩增效果影响很大,浓度过高可降低PCR扩增的特异性,出现非特异性扩增;浓度过低会降低TaqDNA聚合酶的活性,使PCR扩增产物量减少且条带亮度较弱。试验发现当Mg2+浓度在2.0 mmol·L-1时扩增效果最好。
-
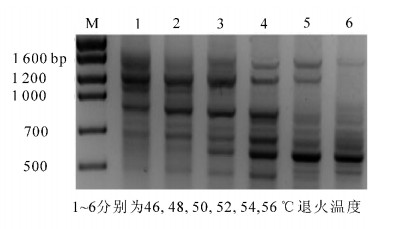
引物的一个重要参数是熔解温度(Tm),手工计算Tm值方法为: Tm=2×(A+T)十4×(G+C) ℃,退火温度一般设定比引物的Tm低5 ℃。本次试验设置了46,48,50,52,54,56 ℃等6个不同温度梯度,对引物ISSR2的退火温度进行优化,其PCR电泳结果见图 6。由图 6可以看出:退火温度对七叶一枝花ISSR-PCR反应影响较大,当退火温度在46~48 ℃时,小片段扩增数量大大降低,并且扩增出的片段模糊不清,当退火温度在52~56 ℃时大片段扩增条带数大大降低,由此可以看出退火温度过低或过高都对PCR扩增不利,并会引起错配现象的发生。当退火温度在52 ℃时扩增出的条带较清晰,且多态性好。因此,通过直观的分析方法确定引物ISSR2的最适退火温度为52 ℃。
-
考虑到各因素之间的交互作用,在单因素试验的基础上筛选出Taq酶,dNTPs,引物和镁离子(Mg2+)4个因素较好的3个水平再进一步进行正交试验。表 2显示的是采用正交设计的9个处理组合。
序号 Taq酶/(×16.67 nkat) dNTPs/ (mmol • L-1) 引物/(μmol•L-1) Mg2+/(mmo•L-1) 平均分 1 0.75 0.15 0.5 1.5 7.33 2 0.75 0.20 0.6 2.0 9.00 3 0.75 0.25 0.7 2.5 8.33 4 1.00 0.15 0.6 2.5 7.66 5 1.00 0.20 0.7 1.5 6.67 6 1.00 0.25 0.5 2.0 8.17 7 1.25 0.15 0.7 2.0 7.00 8 1.25 0.20 0.5 2.5 8.00 9 1.25 0.25 0.6 1.5 7.33 均值1 8.220 7.330 7.833 7.110 均值2 7.500 7.667 7.773 7.833 均值3 7.443 8.167 7.557 8.220 极差 0.777 0.837 0.276 1.110 Table 2. Optimized orthogonal design of ISSR-PCR reaction system for Paria polyphylla
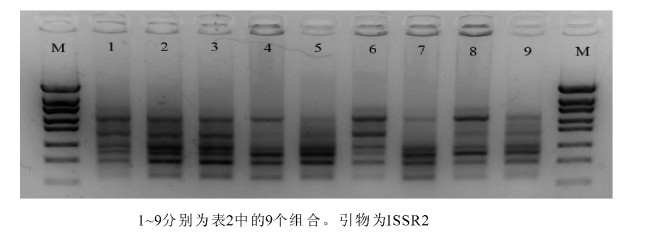
图 7为七叶一枝花ISSR-PCR正交试验扩增结果。由图 7可以看出,9个处理组合均能扩增出条带,但条带之间的效果存在一定差异。
对9个组合进行3次重复试验,根据电泳条带数量、清晰度和背景深度3个指标,对正交试验的9个组合满分10分制进行打分,9个组合3次重复的分数依次为7.0,8.0,7.0;8.0,9.0,8.0; 9.0,9.0,9.0;7.0,8.0,8.0;6.0,7.0,7.0;8.0,8.5,8.0;7.0,7.0,7.0;8.0,8.0,8.0和7.0,8.0,8.0。将其进行极差分析见表 2。极差R值分析可知,4个因素对反应体系影响从大到小的顺序依次为Mg2+,dNTPs,Taq DNA聚合酶和引物;综合平均分值可以看出2号组合综合得分最高,条带数较多且清晰明亮,是较为理想的组合。从扩增的效果及成本投入综合考虑,确定2号组合为最佳选择:即ISSR-PCR 25.0 μL反应体系中,dNTPs 0.2 mmol·L-1,TaqDNA聚合酶0.75×16.67 nkat(0.75 U),引物0.6 μmol·L-1,Mg2+ 2.0 mmol·L-1,模板40 ng。
利用以上获得的ISSR-PCR最佳反应体系进行可靠性检测,对七叶一枝花野外采集的4个居群的21个样品进行PCR扩增,结果见图 8。ISSR2引物在21个样品中均能扩增出清晰可辨、稳定可靠、多态性高的条带,证明该体系适合七叶一枝花样品的ISSR-PCR扩增。
3.1. 七叶一枝花总DNA的提取
3.2. 七叶一枝花ISSR-PCR反应体系的单因素试验优化
3.2.1. 三磷酸碱基脱氧核苷酸(dNTPs)浓度对ISSR-PCR的影响
3.2.2. Taq DNA聚合酶浓度对ISSR-PCR反应的影响
3.2.3. 引物浓度对ISSR-PCR反应的影响
3.2.4. 模板DNA用量对ISSR-PCR反应的影响
3.2.5. Mg2+浓度对ISSR-PCR反应的影响
3.2.6. 引物ISSR2最佳退火温度的选择
3.3. 七叶一枝花ISSR-PCR反应体系的正交优化
-
ISSR分子标记技术是基于稳定而可靠的PCR反应体系的一种技术,ISSR-PCR反应易受TaqDNA聚合酶,模板DNA,dNTPs,引物,镁离子(Mg2+)浓度及退火温度等多种因素的影响。七叶一枝花是重楼属植物,虽然同属其他植物也进行过ISSR分析研究,但所建立的反应体系却各不相同[20-22],说明物种不同,最适的反应条件也存在差异,有必要针对特定物种建立最适的ISSR-PCR反应体系。
本研究在单因素多水平筛选的基础上,进行正交设计试验,既考虑了各因素的最佳条件,又兼顾了因素之间的交互作用,从而筛选出重复性好、多态性高的七叶一枝花最佳ISSR-PCR反应体系,即25.0 μL体系中dNTPs 0.2 mmol·L-1,TaqDNA聚合酶0.75×16.67 nkat(0.75 U),引物0.6 μmol·L-1,Mg2+2.0 mmol·L-1,模板DNA 40 ng,引物ISSR2最适退火温度为52 ℃。该体系的建立为后续的七叶一枝花种质资源鉴定和遗传多样性研究奠定了基础。









 DownLoad:
DownLoad: