-
光照和土壤水分状况直接影响植物的光合作用、形态特征乃至生态适合度[1-2]。一般地,强光与弱光都将产生光胁迫效应。盛夏午间强光会引起植物光合速率出现谷值,严重时导致植物叶片萎蔫甚至枯死;持续处于弱光生境也会使植株光合效率降低、生理生化过程改变[3]。土壤水分含量的变化同样影响植物的光合速率与形态建成,但一定程度的缺水会引起植物更为活跃的光合生理过程[4]。资源类或条件类生态因子的变化与植物光合生理响应受到众多学者的关注,较多研究聚焦于单因子梯度变化对植物产生的生态效应,如不同光照条件下活血丹Glechoma longituba叶绿体超微结构、光合作用和次生代谢产物的积累分析[5],不同土壤水分条件下七叶一枝花Paris polyphylla光合特性及皂苷含量的研究[6]。新近研究关注2个生态因子对植物的复合生态效应,如在弱光环境下,适当水分胁迫可缓解披针叶茴香Illicium lanceolatum栽培过程中弱光胁迫带来的生长限制效应[7];适度遮光与适度干旱协同提高了荩草Arthraxon hispidus的光合能力[8]。设置双因素控制试验,模拟自然生境主要生态因子的复合影响,研究结果更接近于植物对野外条件的生态响应[9-10]。通过叶绿素荧光诱导动力学曲线可分析出大量PSⅡ反应中心原初光化学信息[11]。强光和水分亏缺会降低PSⅡ原初光能转换能力[12];严重的干旱胁迫会使植物叶片O-J-I-P曲线变形为O-K-J-I-P曲线[13]。芒萁Dicranopteris dichotoma属于中国亚热带低山丘陵区次生植被的标志种和识别种[14-16],对土壤条件变化表现出较强可塑性[17],能够密集生长于马尾松Pinus massoniana、杉木Cunninghamia lanceolata等单优群落下层,杜鹃Rhododendron simsii灌丛下层及林缘,形成单优草本层片[18-19],表明芒萁分布地段的光强与水分因子呈现梯度变化,推测单优草本层片的形成与芒萁对光强和土壤水分梯度变化的适应性存在密切的生态关系。由此提出如下猜测,强光下充足的土壤水分可能会减轻芒萁的强光胁迫,而弱光下适当减少土壤水分也可能会减轻芒萁的弱光胁迫。为此,本研究通过设置遮光梯度和土壤水分梯度控制实验,模拟芒萁野外生境,测定盆栽芒萁光合生理参数和叶绿素荧光动力学参数,分析芒萁光合生理过程对光照强度与土壤水分的生态响应特征,探究芒萁生长分布扩散与光强、土壤水分变化的生态关系,为调控芒萁单优层片、促进森林更新提供可借鉴的理论依据。
HTML
-
实验地位于浙江农林大学东湖校区(30°15′28″N,119°43′35″E),全年降水量1 628.6 mm,全年平均气温16.4 ℃,年日照时数1 847.3 h,无霜期约237 d。芒萁幼苗采于杭州市临安区玲珑山(30°13′05″N,119°40′23″E)海拔160 m处,选取长势良好且高度基本一致的幼苗100株,于2018年5月栽植于40 cm(长)×21 cm(宽)×17 cm(高)的长方形花盆内。栽培基质为马尾松单优群落下层表土(田间持水量为27.87%),每盆定植1株。设置3个梯度的遮光大棚,透光率分别为35.96%(轻度遮光,记为L1),13.00%(中度遮光,记为L2)和4.75%(高度遮光,记为L3)。将芒萁幼苗放置于L2遮阳网下进行缓苗。2个月后将成活的盆栽芒萁(简称芒萁)随机分成10组,10 盆·组−1,1组移至全光下作为对照组(ck),其余移至3个遮光强度的大棚内,每个遮光强度下设置3个土壤水分梯度,土壤相对含水量分别为田间持水量的75%~80%(充足水分,记为W1),50%~55%(中度干旱,记为W2),25%~30%(重度干旱,记为W3);ck充足水分处理。土壤水分处理90 d后,测定芒萁光合参数和叶绿素荧光参数。
-
利用Li-6400XT光合测定仪于2018年10月晴天的8:30−11:30(光合有效辐射727~1 229 μmol·m−2·s−1),测定芒萁叶片的光合气体交换参数。测定前,让待测叶片在饱和光(1 500 μmol·m−2·s−1)下照射30 min,使叶片充分适应。设定光合有效辐射梯度依次为2 000、1 500、1 200、1 000、800、500、300、100、50、30、10和0 μmol·m−2·s−1。测定已设定光合有效辐射下的净光合速率(Pn)。每个处理选择3盆芒萁,每盆重复打点3次,计算平均值。利用光合助手软件(Photosynth Assistant)对Pn—PAR光响应曲线进行拟合[20],计算光补偿点(LCP)、光饱和点(LSP)等光合特征参数。拟合方程为:
其中:Pmax为最大净光合速率,Pmax≤50 μmol·m−2·s−1;YAQ为表观量子效率,0<YAQ<0.125 μmol·mol−1;PAR为光合有效辐射;K为光响应曲线的曲率,0<K<1.2;Rd为暗呼吸速率。
-
利用便携式动态荧光仪(YZQ-500),测定不同处理下芒萁成熟叶片的叶绿素荧光动力学曲线(O-J-I-P曲线)。测定前将待测芒萁暗处理40 min,每个处理测3盆,每盆重复测定3次。从O-J-I-P曲线上可以直接获得如下参数:初始荧光FO=F50 μs;J点荧光FJ=F2 ms;I点荧光FI=F30 ms;P点荧光FP=FM。计算得到以下参数[21]:最大光化学效率φPo、J点相对可变荧光VJ、荧光诱导曲线的初始斜率MO、电子传递效率ψO、电子传递量子产额φEo、用于热耗散的量子比率φDo、单位面积吸收的光能ABS/CSM(在t=tFM时)、单位面积内反应中心的数量RC/CSM(在t=tFM时)、单位面积捕获的光能TRO/CSM(在t=tFM时)、单位面积电子传递的量子产额ETO/CSM(在t=tFM时)、单位面积的热耗散DIO/CSM(在t=tFM时)、以吸收光能为基础的性能指数PIABS和综合性能指数PItotal。
-
运用Excel对原始数据进行处理,计算平均值与标准差。借助SPSS 22.0软件,采用单因素方差分析法(one-way ANOVA)对不同处理下芒萁的光合特征参数及叶绿素荧光动力学参数进行差异显著检验,采用最小显著差数法(LSD)进行多重比较分析。利用Origin 2018制图。
1.1. 材料和实验设计
1.2. 测定方法
1.2.1. 光合参数的测定
1.2.2. 叶绿素荧光诱导动力学曲线的测定
1.3. 数据处理方法
-
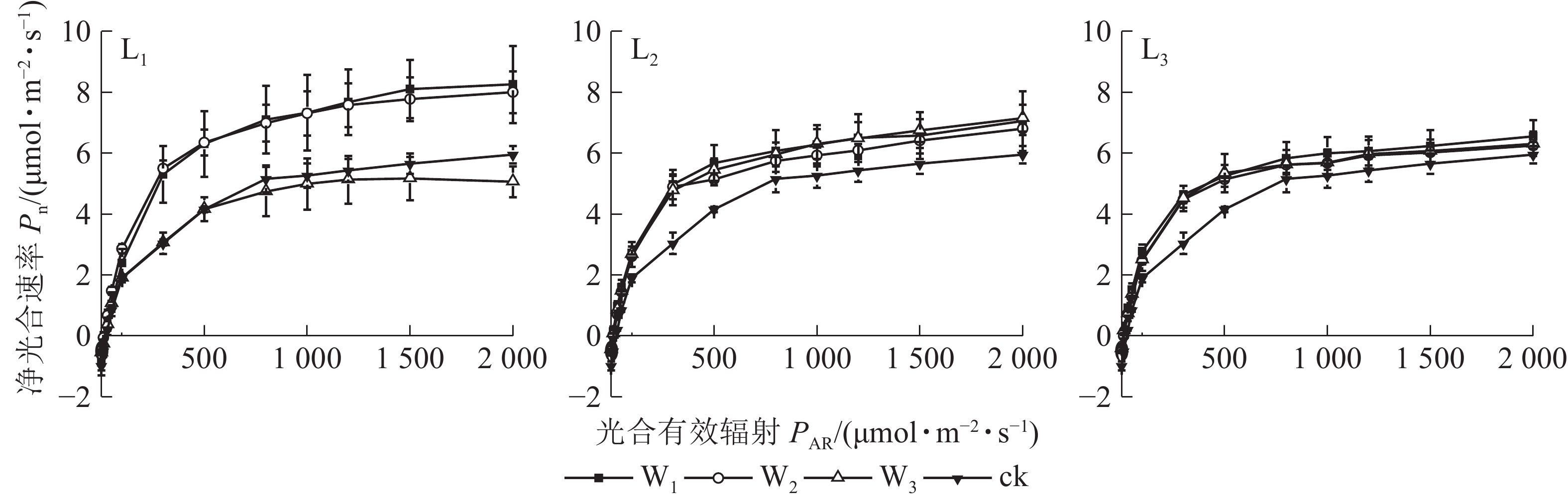
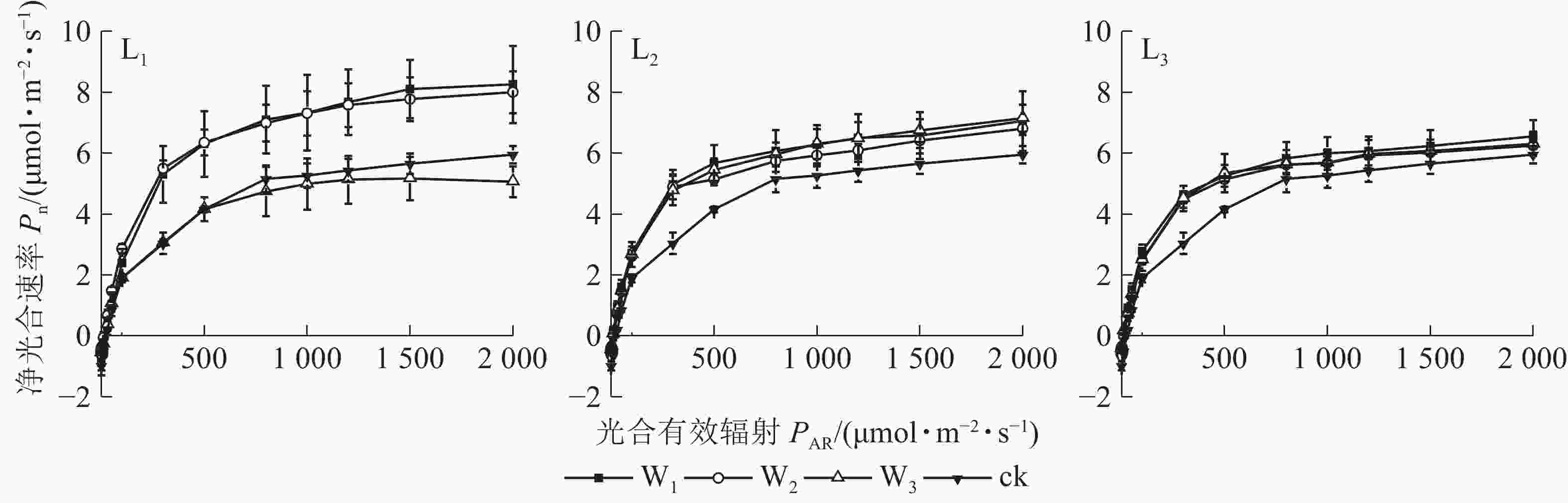
由图1可见:L1遮光处理下,芒萁的Pn变化从大到小依次为W1、W2、W3,其中W1与W2相近;W3的Pn在PAR>100 μmol·m−2·s−1后就趋向平缓,与W1和W2形成明显的差异,PAR>500 μmol·m−2·s−1时,较W2下降超过30%,且低于ck,其余各遮光处理的Pn
均高于ck,说明重度干旱使轻度遮光的芒萁Pn受到显著抑制。L2与L3的芒萁Pn变化大致相同,从大到小依次为W1、W3、W2,不同水分处理间无显著差异(P>0.05)。 -
分析表1可知:L1W1的LSP显著高于其他遮光处理(P<0.05),与ck相比提升了10.5%,表明水分充足且轻度遮光能够提高芒萁的强光利用能力。L1处理下,随土壤水分的减少,芒萁的LSP降低,LCP先降低后升高,重度干旱使芒萁的光强生态幅缩小。遮光显著降低了芒萁的LCP(P<0.05),其最低值出现在L2W1,仅为ck的26.74%。L1遮光处理下,重度干旱显著降低了芒萁的YAQ(P<0.05),比ck低15.9%;L2芒萁的YAQ比ck高22.7%~27.3%,但水分处理之间没有显著差异(P>0.05)。L1芒萁的Pmax随土壤水分减少而降低,其中W1和W2的Pmax显著大于其他处理(P<0.05),分别达到ck的127.9%和120.4%,而W3的Pmax出现最小值,为ck的80.7%;L2和L3芒萁的Pmax差异不显著(P>0.05)。ck和L1W1的Rd
显著高于其他处理(P<0.05);L2W1的Rd为各处理间最低,为ck的41.28%,说明中度遮光和充足水分利于芒萁积累光合产物。总体而言,在L1遮光条件下,土壤水分处理对芒萁LSP、Pmax和YAQ的影响最为显著;而L2、L3处理下干旱胁迫未显著降低芒萁的光合参数。由此推测,干旱胁迫主要降低芒萁的强光利用能力,但不影响弱光利用能力。 遮光 水分 光饱和点
LSP/(µmol·m−2·s−1)光补偿点
LCP/(µmol·m−2·s−1)表观量子效率
YAQ/(µmol·mol−1)最大净光合速率
Pmax /(µmol·m−2·s−1)暗呼吸速率
Rd/(µmol·m−2·s−1)ck 1 037.3±33.0 ab 27.3±1.5 a 0.044±0.006 bc 7.644±0.479 bcd 0.981±0.148 a L1 W1 1 146.2±91.9 a 20.4±10.4 b 0.046±0.006 b 9.780±0.955 a 0.879±0.420 a W2 979.6±174.0 bc 10.7±3.8 c 0.054±0.011 a 9.207±1.128 a 0.614±0.292 b W3 820.7±138.9 de 18.0±7.0 b 0.037±0.012 c 6.172±0.835 e 0.554±0.027 b L2 W1 914.0±138.2 bcd 7.3±1.5 c 0.055±0.003 a 7.805±1.028 bc 0.405±0.065 b W2 892.0±17.9 cde 9.3±1.9 c 0.054±0.005 a 7.505±0.172 bcd 0.486±0.060 b W3 1 021.8±40.0 b 9.3±5.0 c 0.056±0.004 a 8.197±0.167 b 0.448±0.194 b L3 W1 866.2±102.4 de 8.0±3.3 c 0.055±0.003 a 7.357±0.578 cd 0.416±0.144 b W2 829.3±114.9 de 10.0±2.0 c 0.044±0.003 bc 6.976±0.179 cde 0.432±0.073 b W3 786.2±39.8 e 8.9±1.7 c 0.045±0.005 b 6.931±0.295 de 0.413±0.077 b 说明:数值为平均值±标准差。不同小写字母表示同一指标不同处理间差异显著(P<0.05) Table 1. Photosynthetic and physiological parameters of potted D. dichotoma under different shading and soil moisture treatment
-
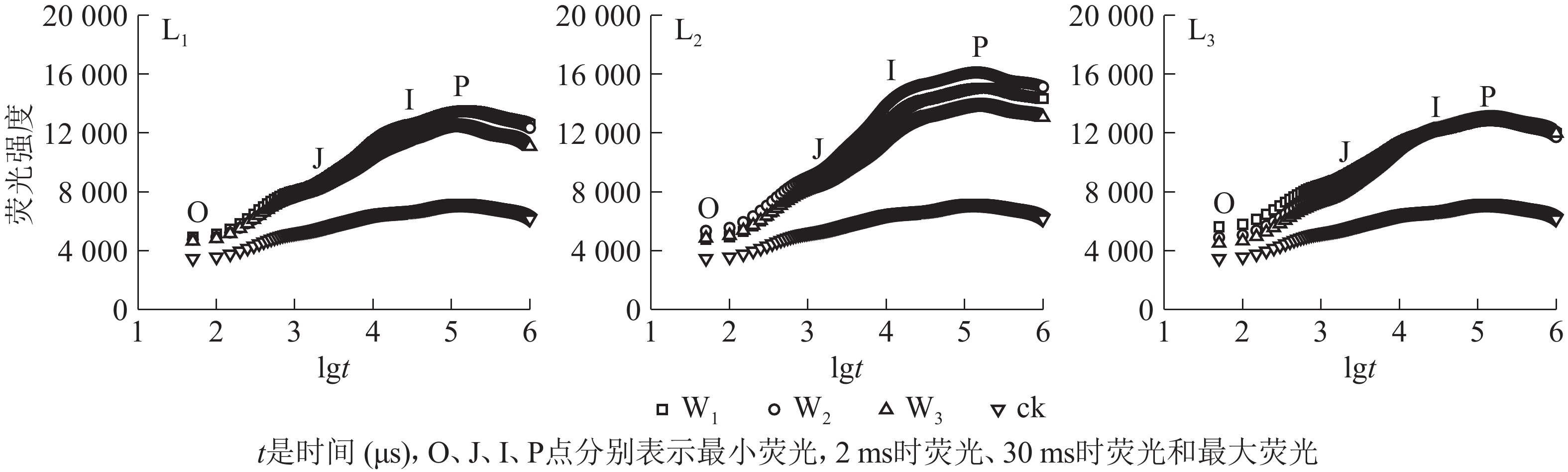
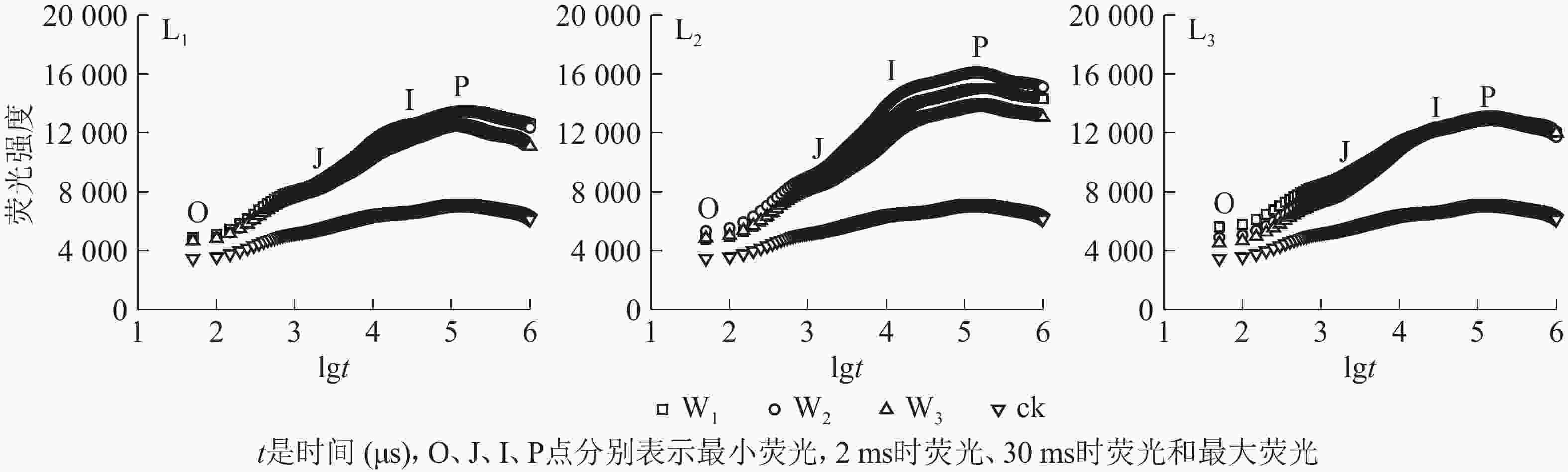
分析图2可知:除ck外,不同遮光和土壤水分处理下芒萁叶片的叶绿素荧光诱导动力学曲线均具有O、J、I、P的典型特征位点,随着遮光强度的增加,荧光强度均在L2遮光处理明显上升,在L3遮光处理下降;ck的荧光曲线较为平缓,没有明显的J、I相,荧光强度明显低于遮光处理。L1遮光处理下,芒萁O-J-I-P曲线荧光强度从大到小依次为W1≈W2、W3,W3处理的芒萁在J-I处的荧光强度升幅低于W1和W2,说明重度干旱影响了芒萁PSⅡ反应中心受体侧初醌受体(QA)的积累。L2遮光处理下,荧光强度从大到小依次为W2、W1、W3,W2处理下的芒萁叶片获得最大的荧光产量。L3遮光处理下,芒萁在O点的荧光强度从大到小依次为W1、W2、W3,但在I点后荧光强度趋于相同,说明该光强条件下PSⅡ主要受弱光的抑制。
-
由表2可知:ck的VJ最高,说明强光引起芒萁PSⅡ的活性反应中心关闭;L1和L2遮光处理下VJ的峰值出现在W3,而L3遮光处理下VJ的峰值出现在W1,即高度遮光的芒萁受到干旱胁迫时PSⅡ活性反应中心关闭程度减轻。MO代表QA被还原的最大速率,L3W1的MO在各处理中最高,说明该处理下QA被还原的速率加快,同时PSⅡ向下游电子传递链传递电子的能力降低;MO较低的处理分别是L1W2、L2W1、L2W2、L3W3,说明适度遮光和中度干旱时芒萁叶片的光合反应中心较为活跃,高度遮光时干旱胁迫提高了芒萁PSⅡ的活性。φPo等同于Fv/Fm,在L1和L2处理下,不同水分处理间芒萁的φPo差异不显著(P>0.05);L3处理中,芒萁的φPo随土壤水分的减少而升高,说明弱光下水分胁迫提高芒萁PSⅡ原初光能转换,推测遮光导致水分利用率提高,从而引起芒萁的抗旱性响应;ck的φPo显著小于各遮光处理(P<0.05),说明全光下芒萁的PSⅡ出现严重的光抑制。ψO和φEo反映PSⅡ受体侧的变化,L1和L2遮光处理下,W3芒萁叶片的ψO和φEo相比W1和W2出现了下降趋势,但在L3下ψO和φEo随土壤水分减少而升高。热耗散的量子比率φDo在L1下差别不大,L2下变化从大到小依次为W3、W2、W1,L3下为W1、W2、W3;ck的φDo远高于各遮光处理,说明全光下芒萁叶片启动了相应的光防御机制,以热能途径耗散过剩激发能,从而减轻强光伤害。
遮光 水分 VJ MO φPo ψO φEo φDo ck 0.564±0.058 a 0.952±0.095 a 0.635±0.035 ab 0.436±0.058 c 0.224±0.055 c 0.496±0.066 a L1 W1 0.433±0.057 bc 0.740±0.159 bc 0.646±0.034 ab 0.567±0.057 ab 0.362±0.052 a 0.365±0.035 cd W2 0.426±0.054 c 0.648±0.107 c 0.623±0.051 ab 0.574±0.054 a 0.373±0.049 a 0.354±0.034 cd W3 0.470±0.080 bc 0.784±0.213 abc 0.683±0.042 a 0.530±0.080 ab 0.334±0.075 ab 0.377±0.051 cd W1 0.424±0.052 c 0.632±0.146 c 0.667±0.056 ab 0.576±0.052 a 0.395±0.055 a 0.317±0.049 d L2 W2 0.440±0.065 bc 0.655±0.205 bc 0.634±0.089 ab 0.560±0.065 ab 0.377±0.068 a 0.333±0.056 cd W3 0.472±0.112 bc 0.738±0.264 bc 0.550±0.071 cd 0.528±0.112 ab 0.345±0.104 ab 0.384±0.098 cd W1 0.498±0.065 ab 0.830±0.149 ab 0.602±0.083 bc 0.502±0.065 bc 0.281±0.070 bc 0.408±0.039 ab L3 W2 0.464±0.065 bc 0.744±0.208 bc 0.648±0.041 ab 0.536±0.065 ab 0.328±0.081 ab 0.403±0.099 bc W3 0.421±0.032 c 0.635±0.126 c 0.504±0.066 d 0.579±0.032 a 0.376±0.042 a 0.352±0.041 cd 说明:数值为平均值±标准差。不同字母表示同一指标不同处理间差异显著(P<0.05) Table 2. VJ
, MO and the yields or flux ratios in leaves of potted D. dichotoma under different shading and soil moisture treatment -
分析表3可知:L2遮光处理下芒萁的RC/CSM处于较高水平,说明中度遮光下芒萁叶片对光能的利用能力较强;ck的RC/CSM显著低于遮光处理,说明遮光后芒萁叶片通过增加有活性的反应中心数量来维持基本的光合过程。L2遮光处理下,芒萁的ABS/CSM、TRO/CSM和ETO/CSM均明显高于L1和L3,说明芒萁PSⅡ系统在L2光强下拥有最高的活性;不同水分处理间上述参数以W2为最高,说明L2W2的水分与光强最能激发芒萁PSⅡ的活性。ck芒萁单位面积叶片的热耗散DIO/CSM相对各遮光处理较低,主要原因是ck芒萁单位面积吸收的光能处于较低水平。遮光处理中,DIO/CSM在L3W1出现最大值,推测对弱光的利用效率低下导致热耗散;其他各处理芒萁的DIO/CSM差异较小。因此,芒萁单位面积各能量流参数在光照梯度间存在明显差异,但在土壤水分处理间差异不显著(P>0.05)。
遮光 水分 RC/CSM ABS/CSM TRO/CSM ETO/CSM DIO/CSM ck 2 223.5±847.4 c 7 194.2±2 013.2 d 3 756.3±1 462.5 d 1 711.5±825.2 c 3 437.8±568.1 c L1 W1 5 109.7±984.1 b 13 513.8±2 288.7 bc 8 596.9±1 606.4 bc 4 912.3±1 180.7 ab 4 916.9±867.8 ab W2 5 806.5±1 315.4 ab 13 482.6±2 359.9 bc 8 744.2±1 733.9 abc 5 099.3±1 370.1 ab 4 738.3±817.2 b W3 4 850.0±1 145.4 b 12 426.4±1 370.0 c 7 762.7±1 141.7 c 4 174.1±1 099.4 b 4 663.8±762.7 b W1 7 112.5±1 287.6 a 15 061.4±1 166.6 ab 10 305.1±1 157.9 ab 5 980.8±1 043.8 a 4 756.3±561.9 b L2 W2 7 581.4±1 507.0 a 16 112.8±967.6 a 10 766.0±1 177.7 a 6 101.8±1 216.1 a 5 346.8±843.4 ab W3 6 116.3±2 225.9 ab 13 815.4±2 180 abc 8 943.0±2 429.1 abc 4 966.6±1 990.4 ab 4 872.4±436.3 ab W1 4 509.7±1 958.7 b 12 909.0±3 402.0 bc 7 289.6±2 666.1 c 3 812.8±1 789.1 b 5 619.4±931.8 a L3 W2 5 402.1±2 449.3 b 12 860.4±3 576.2 bc 7 971.1±2 875.1 c 4 423.3±1 905.1 b 4 889.3±1 031.1 ab W3 5 803.6±1 397.4 ab 12 981.7±1 838.6 bc 8 457.3±1 462.9 bc 4 918.6±962.2 ab 4 524.3±585.0 b 说明:数值为平均值±标准差。不同字母表示同一指标不同处理间差异显著(P<0.05) Table 3. Density of RCs and phenomenological energy fluxes per CS in leaves of potted D. dichotomaunder different shading and soil moisture treatment
-
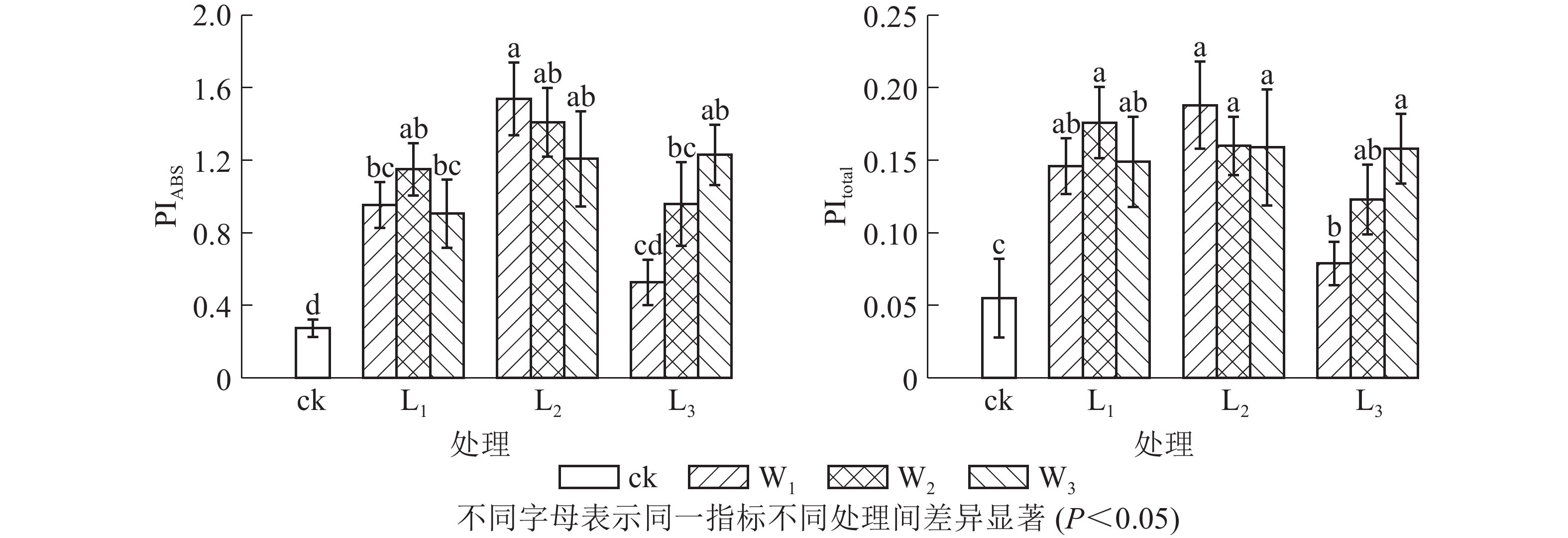
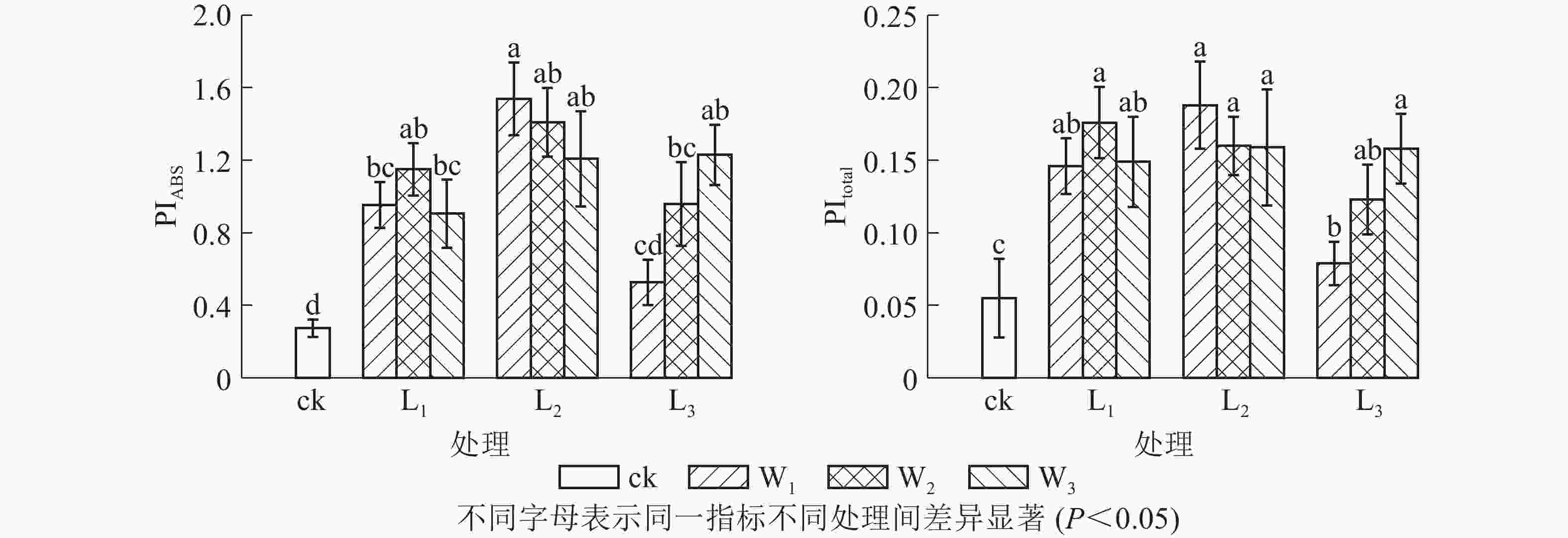
PIABS是吸收光能的性能指数,比Fv/Fm(φPo)能更准确地反映植物光合机构的状态。由图3可见:L2遮光处理的芒萁光合机构能力最强,且随土壤水分的减少而下降,中度和重度干旱处理分别比充足水分处理下降8.38%和21.51%。L3不同水分处理的PIABS差异显著(P<0.05),意味着弱光下芒萁对土壤水分变化更敏感。PItotal主要用于研究光系统间的电子传递活性,可进一步反映胁迫对植物叶片光系统Ⅰ的影响。PItotal的变化趋势大致上与PIABS相同。L3W1光强水分处理芒萁的光合活性较低,弱光下光系统Ⅰ的调节能力较弱。
2.1. 遮光和土壤水分处理对芒萁光响应曲线的影响
2.2. 遮光和土壤水分处理对芒萁光合特征参数的影响
2.3. 遮光和土壤水分处理对芒萁叶绿素荧光诱导动力学曲线的影响
2.4. 遮光和土壤水分处理对芒萁叶绿素荧光诱导动力学参数的影响
2.4.1. 遮光和土壤水分处理对芒萁叶片VJ、MO和量子产额的影响
2.4.2. 遮光和土壤水分处理对芒萁叶片单位横截面反应中心密度和能量流参数的影响
2.4.3. 遮光和土壤水分处理对芒萁叶片光合性能指数(PIABS)和综合性能指数(PItotal)的影响
-
遮光和干旱对芒萁的气体交换参数的影响具有明显的协同作用。在L1遮光处理下,重度干旱使芒萁的Pn
-PAR曲线在100 μmol·m−2·s−1后就趋向平缓。由此说明:重度干旱降低了芒萁在较强光照下的光合能力;L2和L3遮光处理下,不同水分处理的光响应曲线的拐点均出现在300~500 μmol·m−2·s−1,重度干旱处理下没有出现Pn明显降低的趋势,说明中度遮光和高度遮光能够缓解水分亏缺对芒萁的Pn的负面影响。 植物的光饱和点和光补偿点是衡量植物耐荫性的重要指标。本研究中,芒萁的光饱和点为786.2~1 146.2 μmol·m−2·s−1,高于其他蕨类植物[22],验证了芒萁喜光的习性[23];光补偿点变化为7.3~27.3 μmol·m−2·s−1,符合耐荫植物的特征[24-25]。此研究结果与前期对芒萁光合能力的研究结果相似[26]。L1W1处理的芒萁LSP和Pmax高于ck,说明L1遮光处理能够为芒萁提供足够的光能,并提高芒萁的光合潜力;同时ck和L1W1的LCP较高,Rd显著高于其他处理(P<0.05),意味着弱光利用率低,消耗碳水化合物较多,因此芒萁虽具有一定的喜光性,但强光环境却不利于其生长。弱光条件下,芒萁通过降低LSP和LCP来减少对光能的需求,降低Rd
使呼吸作用造成的碳损耗保持较低水平,以此维持碳平衡[25]。L2W1处理下芒萁的LCP和Rd为各处理中最低,说明中度遮光和充足水分条件最有利于芒萁积累光合产物。 YAQ越大,植物的耐荫性越强[27]。大多数植物的YAQ的实测值为0.03~0.05[28]。本研究中,L2遮光处理下的芒萁YAQ均大于0.05,说明中度遮光下芒萁具有较强的耐荫性;只有L1W3的芒萁YAQ小于ck,结合LSP、LCP和Pmax等参数,可以认为强光和重度干旱组合不利于芒萁光合作用。
-
叶绿素荧光动力学分析能够反映植物光合机构内部的调节过程。不同处理下芒萁荧光动力学曲线均无明显K相,说明芒萁PSⅡ的电子供体侧和受体侧未受到严重破坏[13]。φPo下降意味着光抑制的产生[29]。缺水和强光均可能导致光抑制。L3遮光条件下,W1的φPo小于W2和W3,说明弱光下充足水分对芒萁造成类似于光抑制的作用。由此推测,芒萁在弱光环境下最适土壤水分较低。一般来说,耐荫性强的植物在强光下的光抑制作用比较明显[30]。本研究中的芒萁符合耐荫性强的植物特征。
L1和L2遮光处理下,W3的芒萁相比W1和W2出现VJ上升,ψO下降的变化特征,同时φEo下降,为消耗过多激活的电子,φDo随之升高。由此可知:轻度和中度遮光下,重度干旱抑制了芒萁叶片供体侧电子传递和PSⅡ受体侧电子传递,降低整个电子传递链效率[31];L3遮光条件下则是W1的VJ最高,可以看出高度遮光下干旱未抑制芒萁PSⅡ活性,推测主要限制因素转变为光强。L1W3和L3W1的芒萁MO较高,受到的胁迫相对严重,反应中心部分降解或失活[32],抑制了PSⅡ受体侧的电子初级受体QA向二级受体QB的电子传递[33]。与L1和L3相比,L2遮光处理下芒萁的ψO、φEo和能量流参数较高,叶片单位面积吸收的光能减少,而用于电子传递的能量增多,比活性较高[34]。因此,L2是芒萁较为适宜的光强,而W2下芒萁叶片的反应中心密度和能量流参数最高,即中度遮光与中度干旱下芒萁拥有最高的PSⅡ活性。土壤水分缺失会导致DIO/CSM增加,这种热耗散现象作为光保护机制出现[35],但本研究干旱胁迫下芒萁的DIO/CSM并未大幅提高,说明在土壤水分含量较低时芒萁能够维持相对稳定的光能利用率。
L3遮光条件下,芒萁的ψO、φEo与ABS/CSM、TRO/CSM、ETO/CSM均在W1处最低,W3处最高,而在L1和L2遮光条件下,上述参数均在W1或W2处最高,W3处最低。可以看出,高度遮光下芒萁PSⅡ增强了对干旱胁迫的适应能力,推测光照强度降低后,芒萁最适土壤水分含量在降低。
光合性能指数PIABS综合反映光系统的活性[36],与φPo相比对干旱胁迫的程度更敏感[37]。综合性能参数PItotal能反映电子在PSⅡ和PSⅠ之间的传递能力及PSⅠ的相关性能[38]。对比PIABS和PItotal发现:两者变化规律相似,说明各处理芒萁荧光诱导曲线的J、I、P三相较为稳定,意味着各处理的芒萁PSⅡ没有受到严重的胁迫,这是芒萁对遮光环境和土壤水分条件适应性较强的体现。
鉴于本研究以盆栽芒萁分株为材料,与自然条件下的适应性表现存在一定差异,因此未来的研究尺度应拓宽至芒萁克隆分株对甚至基株系统,并通过构建侧方遮光、光斑及缝隙透光类型与土壤主要养分、酸度等组合试验,使研究结果更接近野外生境,围绕芒萁单优层片发育机制开展更深入的研究。
3.1. 遮光和土壤水分处理对芒萁光响应进程的影响
3.2. 遮光和土壤水分处理对芒萁叶绿素荧光的影响
-
芒萁气体交换参数、叶绿素荧光参数均对遮光处理和干旱胁迫响应明显,表现出较强的耐荫性和耐旱能力。轻度遮光条件下,重度干旱显著降低了芒萁的Pn、YAQ和Pmax(P<0.05),充足水分(W1)和中度干旱(W2)的Pn大约比重度干旱(W3)和ck增加34.4%~69.2%。在中度遮光和高度遮光条件下,各控水处理之间芒萁的Pn、LCP和Rd差异不显著(P>0.05),遮光程度增加能够缓解水分亏缺对芒萁净光合速率的负面影响。中度遮光+充足水分组合处理芒萁的LCP和Rd为各处理中最低,有利于积累光合产物。
相同水分处理条件下,随着遮光程度增加,芒萁的荧光参数多呈现先增加后下降的变化特征。芒萁叶片单位横截面积反应中心密度和能量流参数显示:轻度和中度遮光下重度干旱降低了芒萁的光合机构能力;中度遮光与中度干旱下芒萁拥有最高的PSⅡ活性。高度遮光时,随土壤水分的降低,芒萁叶片的ψO、φEo、能量流参数和PIABS均出现上升趋势,表现出明显的抗旱性,弱光下适当减少土壤水分可以减轻芒萁的弱光胁迫。遮光和干旱处理未对芒萁的PIABS和PItotal产生明显的抑制作用,结合叶绿素荧光诱导曲线分析可得,芒萁的PSⅡ未受到严重损害,芒萁对光强和土壤水分变化表现出较强的适应性。









 DownLoad:
DownLoad: