-
白菜Brassica campestris ssp. chinensis是十字花科Brassicaceae芸薹属Brassica 2年生草本植物,因成熟周期短、适应力强、营养价值高等特点,在中国农业生产中占据重要地位[1]。硫代葡萄糖苷简称硫苷,是十字花科植物中的一类含硫、氮次生代谢产物,基于衍生氨基酸的差异可分为脂肪族、吲哚族和芳香族三大类[2-3]。硫苷的生物合成可分为侧链延伸、核心结构形成和侧链修饰等3步,涉及大量的基因、酶和转录调节因子[4]。已有研究表明:硫苷不仅在抗癌[5]、十字花科植物特殊风味的产生[6-7]及硫元素的调节[8]中发挥着重要的作用,而且是十字花科植物抵御生物胁迫的主要防御物质[9-10]。研究表明:硫苷可以有效影响害虫的生长发育及产卵[11],但不同类型的硫苷对不同种群植食性昆虫抗性不同[12-13]。如甘蓝夜蛾Mamestra brassicae幼虫在取食脂肪族和吲哚族硫苷含量较高的植物后生长缓慢,取食吲哚族硫苷含量较高的植物后发育时间也会延长[14]。作为一种防御相关植物次生代谢产物,硫苷存在于植物的整个生命周期中[15-16],植食性昆虫的取食会使其含量显著提高[17-18],硫苷含量的改变被证明与害虫胁迫下硫苷的合成、转运及降解相关基因的激活相关[19]。MEWIS等[20]发现拟南芥Arabidopsis thaliana受桃蚜Myzus persicae、甘蓝蚜Brevicoryne brassicae和甜菜夜蛾Spodoptera exigu幼虫取食后,脂肪族硫苷含量显著增加,AtMAM1和AtCYP79F1基因转录水平也显著上调。此前,KOORNNEEF等[21]发现特定硫苷生物合成基因的存在或缺失可以赋予某些植物特殊的抗性,如拟南芥Ler生态型AtMAM2的存在,使得其对甜菜夜蛾幼虫的抗性高于缺乏AtMAM2的拟南芥Col生态型。同样,蚜虫侵袭会使拟南芥吲哚族硫苷含量增加3倍[22],PFALZ等[23]通过数量性状基因位点(QTL)定位结合转录组分析,发现吲哚族硫苷合成相关基因AtCYP81F2的表达有助于拟南芥抵抗桃蚜,但不能抵御小菜蛾Plutella xylostella 、欧洲粉蝶Pieris brassicae、粉纹夜蛾Trichoplusia ni、甜菜夜蛾等鳞翅目Lepidopteran害虫。在进化过程中,昆虫为应对植物硫苷-黑介子酶防御系统的防御作用,也形成了以解毒酶——细胞色素P450s和谷胱甘肽硫转移酶(GSTs)等为基础的适应性机制[24−25]。
本研究以白菜为研究对象,通过高效液相色谱法(HPLC)和实时荧光定量聚合酶链式反应(RT-qPCR),探究白菜硫苷质量摩尔浓度及组分在甜菜夜蛾幼虫取食胁迫下的变化与潜在的分子调控机制,并通过检测取食前后甜菜夜蛾幼虫体内解毒酶活性的变化,探究白菜硫苷质量摩尔浓度的提高对幼虫体内解毒酶活性的影响,以期为白菜等十字花科作物应对害虫胁迫抗性的提高、品质和产量的改良提供依据。
-
本研究所用白菜品种为‘美都黑油筒’B. campestris ssp. chinensis ‘Meiduheiyoutong’,培养条件为光照(28 ℃/14 h,30 000 lx)与黑暗(26 ℃/10 h)循环。挑选长势一致的“八叶一心”时期白菜的第5、6片真叶进行接虫处理。研究所用甜菜夜蛾3龄幼虫由浙江农林大学植物保护课题组提供,接虫前将甜菜夜蛾3龄幼虫饥饿处理4 h,每片叶接虫1头。
-
剪取白菜叶片,去除大叶脉,于液氮中冷冻后放入−80 ℃冰箱中保存备用(接虫叶片在取样前用酒精棉将接虫叶片上的排泄物擦拭干净)。将取食后1、24、48和72 h的甜菜夜蛾幼虫放置在冰上冻僵,用解剖针取其中肠,保存在−20 ℃冰箱中备用。
-
参考KRUMBEIN等[26]经过ZHU等[27]修改的方法提取硫苷。称0.25 g样品粉末,加入75 ℃预热的体积分数为70%甲醇溶液4 mL,同时加入200 μL 5 mmol·L−1的2-丙烯基硫苷作为内标,混匀后放在75 ℃水浴锅中水浴10 min,间隔震荡;6 000 r·min−1 离心10 min收集上清液,剩余沉淀则继续加入2次3 mL 75 ℃预热的体积分数为70%的甲醇提取、涡旋、离心,将3次收集的上清液合并倒入10 mL容量瓶中定容;滤纸进行粗过滤,取5 mL过滤液装载到DEAE Sephadex A25固相萃取柱,待滤液全部流完后,加6 mL纯净水清洗柱子,再加入250 μL硫酸酯酶,30 ℃反应12 h,5 mL纯净水洗脱,洗脱液用0.45 mm滤膜过滤后在−20 ℃下保存。HPLC分析进样量20 μL,C-18反相柱柱温30 ℃,流动相为超纯水和乙腈,1 mL·min−1,0~45 min内乙腈质量浓度线性梯度0%~20%,检测波长为229 nm。
-
采用Trizol法提取白菜总RNA,提取过程中防止RNase污染。具体步骤为:取0.2 g植物组织材料快速研磨至粉末状,迅速将其移入装有1 mL预冷Trizol的离心管中,间隔震荡。4 ℃ 12 000 r·min−1离心10 min,取1 mL上清液,加入200 μL预冷氯仿,振荡混匀,冰上静置3 min;4 ℃ 12 000 r·min−1离心10 min,取500 μL上清液,加入100 μL预冷氯仿,振荡混匀,冰上静置3 min;4 ℃ 12 000 r·min−1离心10 min,取400 μL上清液至新的离心管,加入等体积预冷的异丙醇,颠倒混匀,−20 ℃冰箱中静置30 min。4 ℃ 12 000 r·min−1离心10 min,弃上清液,加入1 mL预冷的体积分数为75%乙醇;4 ℃ 13 000 r·min−1离心5 min,弃上清液,加入20 μL 预冷的体积分数为0.1%的无核酸酶水溶解沉淀,−20 ℃保存备用。用NanoDrop 2000检测RNA样品的浓度后,用质量浓度为1.2%的琼脂糖凝胶电泳检测RNA样品的质量。cDNA第1链的合成参照Takara公司的反转录试剂盒(Prime ScriptTM RT reagent Kit with gDNA Eraser)的说明书进行。
-
以反转录获得的cDNA为模板,引物由Primer 5软件设计,以白菜BcUBC10基因[28]作为内参,基因的定量引物见表1。荧光定量PCR扩增体系为:2×SYBR Premix Ex-TaqTM 10 μL,10 ng·μL−1cDNA 1 μL,上下游引物各0.3 μL,无核酸酶双蒸水(RNase free ddH2O) 补足至15 μL。反应循环条件:95 ℃预变性30 s,95 ℃变性5 s,60 ℃复性34 s,共40个循环,每份3次重复。用
$2^{-\Delta\Delta C_{t}}$ 法计算其相对表达量[29],琼脂糖凝胶电泳检测反应产物。基因名称 上游引物(5 '→3 ') 下游引物(5 '→3 ') BcMAM1 CCAGAGTACATACCGCACAA AGAGGACACCGGAAAACCAA BcCYP83A1 AGTTCTCCTCTTCTTCCTCT CCATTGTTTGACTTCCTATC BcCYP83B1 ACACTTCCTCTTTCGTCTCT CATCGTTTGTTGCCCCTTCA BcMYB28 GAGAATTTGCATTCCCTTGC TGGTGTCCCATCTTTGTTGG BcSOT16 GGGTTATGGGTTTAGTGCTG CTCCAACCTTCCCTTTCCTA BcUBC10 GGGTCCTACAGACAGTCCTTAC ATGGAACACCTTCGTCCTAAA BcAPK2 CAACACCGTCTGGGATCTGC AACCGACATCGACACCTGGA BcSUR1 GGCGTTATCTACATGCTGTTCG CAGGTGCGGAAGCAAGGGTA BcAOP2 TGGATTTGCACCAAAGGAAA AGATAGATCACTGGAAGTTG BcBCAT4 AAGCAACTCGACTCAAACACT CGATAAAACCCGAATCCTAAT Table 1. Primer sequences for RT-qPCR analysis
-
根据GSTs试剂盒说明书(南京建成生物有限公司)进行。
-
采用Excel 2020进行数据整理与分析,所有数据均是至少3次生物学重复的平均值±标准误;采用 SPSS 22.0进行差异显著性分析,P<0.05为差异显著。
-
在白菜中共检测到8种硫苷组分(表2),其中:脂肪族3种、吲哚族4种、芳香族1种。如表3所示:甜菜夜蛾幼虫取食不同时间点(1、24、48、72 h)白菜硫苷质量摩尔浓度呈显著增加趋势(P<0.05),取食1和48 h时增幅最大,72 h达峰值(2.03 µmol·g−1),是对照的1.8倍;吲哚族和脂肪族硫苷占总硫苷比例最高。甜菜夜蛾幼虫取食后两者显著增加(P<0.05),并分别在取食48和72 h时达到峰值,是对照的1.6和2.4倍;取食1和24 h时芳香族硫苷质量摩尔浓度也呈显著(P<0.05)上升趋势。这一结果表明甜菜夜蛾幼虫的取食能够诱导白菜硫苷的合成,其中吲哚族硫苷变化最为剧烈,说明吲哚族硫苷可能在抵御鳞翅目昆虫甜菜夜蛾幼虫取食过程中发挥重要的作用。3-吲哚基甲基硫苷(GBC)和1-甲氧基吲哚-3-甲基硫苷(NEO)在甜菜夜蛾幼虫取食后不断增加,在取食72 h时达峰值,分别是对照的4.7和4.9倍;而4-戊稀基硫苷(GBN)、4-羟基吲哚-3-甲基硫苷(4OH)、2-羟基-3-丁烯基硫苷(PRO)和4-甲氧基吲哚-3-甲基硫苷(4ME)则呈先增后减趋势,其中GBN对甜菜夜蛾幼虫取食的响应最为强烈,48 h达峰值,是对照的1.9倍;3-丁烯基硫苷(GNA)和2-苯乙基硫苷(NAS)变化不明显,仅在初始时间点有所增加。
化合物名称 缩写 所属类别 分子量 保留时间/min 2-羟基-3-丁烯基硫苷progoitrin PRO 脂肪族 388 7.790 3-丁烯基硫苷gluconapin GNA 脂肪族 372 14.480 4-羟基吲哚-3-甲基硫苷4-OH-glucobrassicin 4OH 吲哚族 463 17.067 4-戊烯基硫苷glucobrassicanapin GBN 脂肪族 386 22.087 3-吲哚基甲基硫苷glucobrassicin GBC 吲哚族 447 26.753 2-苯乙基硫苷gluconasturtiin NAS 芳香族 422 31.497 4-甲氧基-3-吲哚基甲基硫苷4-methoxy-glucobrassicin 4ME 吲哚族 477 32.433 1-甲氧基-3-吲哚基甲基硫苷1-methoxy-glucobrassicin NEO 吲哚族 477 40.793 Table 2. Components of glucosinolate in B. campestris ssp. chinensis
类型 化合物缩写 硫苷质量摩尔浓度/(µmol·g−1) 0 (ck) 1 24 48 72 h 脂肪族 GNA 0.154±0.010 b 0.174±0.005 b 0.220±0.003 c 0.132±0.003 a 0.132±0.002 a PRO 0.152±0.003 a 0.150±0.002 a 0.176±0.001 b 0.182±0.002 c 0.179±0.001 bc GBN 0.434±0.032 a 0.466±0.009 a 0.550±0.018 b 0.819±0.004 c 0.781±0.001 c 总脂肪族 0.724±0.009 a 0.792±0.001 b 0.959±0.008 c 1.132±0.002 e 1.116±0.004 d 吲哚族 4OH 0.157±0.012 a 0.177±0.003 b 0.222±0.004 c 0.257±0.006 d 0.234±0.004 c GBC 0.044±0.013 a 0.062±0.000 b 0.105±0.003 c 0.130±0.001 d 0.209±0.005 e 4ME 0.089±0.026 a 0.69±0.000 a 0.140±0.003 b 0.154±0.002 b 0.135±0.000 b NEO 0.054±0.011 a 0.067±0.001 b 0.092±0.001 c 0.162±0.003 d 0.263±0.001 e 总吲哚族 0.351±0.001 a 0.375±0.001 b 0.565±0.005 c 0.703±0.010 d 0.840±0.008 e 芳香族 NAS 0.096±0.004 ab 0.109±0.002 c 0.142±0.001 d 0.086±0.001 a 0.101±0.002 bc 总硫苷 1.154±0.003 a 1.277±0.002 b 1.664±0.014 c 1.921±0.011 d 2.032±0.014 e 说明:同行不同小写字母表示同一指标不同时间差异显著(P<0.05) Table 3. Effects of feeding on the content of glucosinolate in B. campestris ssp. chinensis by S. exigua larvae
-
通过RT-qPCR对甜菜夜蛾幼虫取食后白菜硫苷合成相关基因表达情况进行分析。结果(图1)表明:甜菜夜蛾幼虫取食后,脂肪族硫苷合成相关基因BcBCAT4、BcMAM1、BcCYP83A1、BcMYB28、BcSUR1的表达量表现出相同的规律,即取食1 h其表达量显著上调(P<0.05),48 h基因表达水平达到最高,而白菜吲哚族硫苷合成相关基因BcCYP83B1表现出相反的变化趋势;脂肪族硫苷合成相关基因BcAOP2和吲哚族硫苷合成相关基因BcSOT16随取食时间的延长表达量逐渐增加,72 h达最高,分别是对照的4.2和3.7倍。此外,硫苷合成过程中的直接硫供体谷胱甘肽(GSH)合成相关基因BcAPK2转录水平呈显著增加趋势,72 h时比对照增加了4.7倍。
-
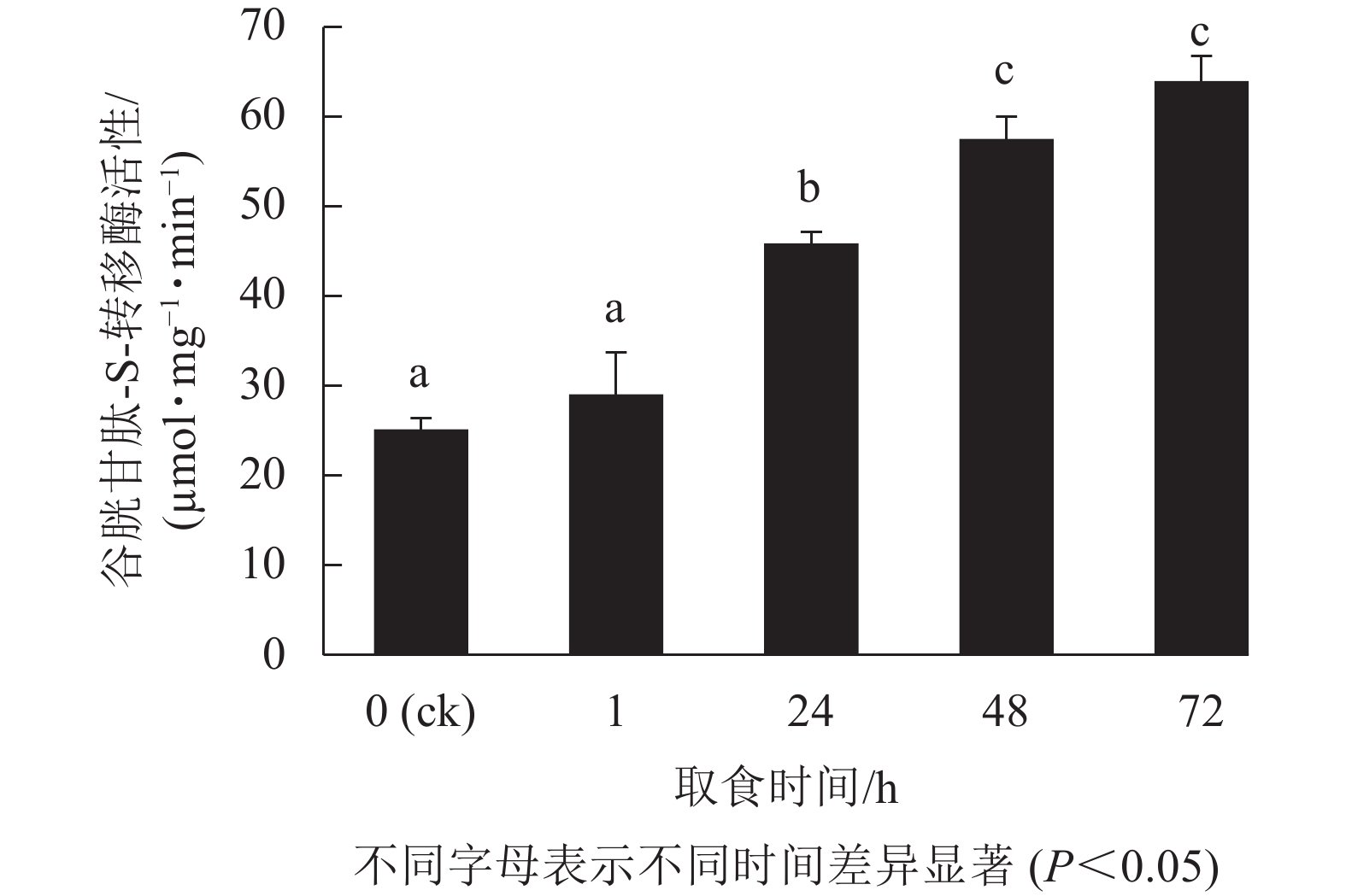
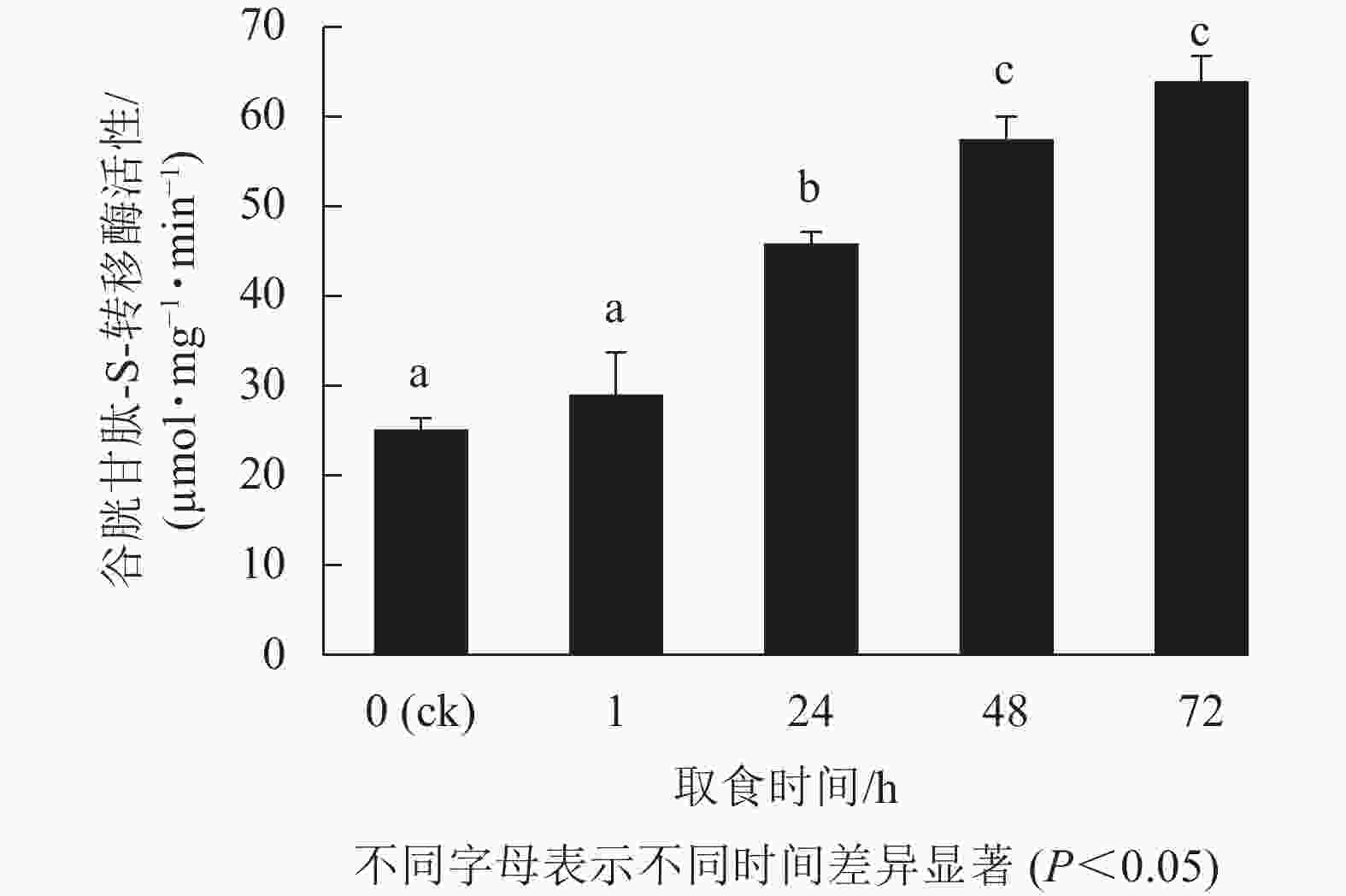
由图2表明:甜菜夜蛾幼虫取食后,幼虫体内GSTs活性产生显著变化,取食1 h时酶活性增强不显著(P>0.05),但在随后的时间点酶活性显著增强(P<0.05),24 h增幅最为剧烈,72 h达峰值,为63.6 µmol·mg−1·min−1,比对照升高了1.5倍。可见,白菜硫苷质量摩尔浓度的增加诱导了甜菜夜蛾幼虫体内解毒酶GSTs活性的提高。
-
已有研究发现:不同拟南芥群体硫苷的种类和含量有所差异,而这种差异影响着拟南芥对不同种群植食性昆虫的抗性[30-33],如甘蓝夜蛾和菜青虫Pieris rapae在取食脂肪族和吲哚族硫苷含量较高的植株时,生长缓慢,取食吲哚族硫苷含量较高的植株时,发育时间也会增加[34]。随后,在研究拟南芥脂肪族硫苷的抗虫机制中,发现GS-Elong、GS-AOP、TGGs等位点与脂肪族硫苷的抗虫性相关[35-38]。SONTOWSKI等[19]发现:拟南芥被甜菜夜蛾幼虫取食后,GS-Elong位点硫苷合成相关基因AtMAM1、AtMAM2、AtMAM3转录水平显著增加,同时相关脂肪族硫苷含量也显著上升,说明脂肪族硫苷参与了拟南芥对甜菜夜蛾幼虫的防御反应。本研究发现:白菜被甜菜夜蛾幼虫取食后,脂肪族硫苷GS-Elong位点相关基因BcBCAT4、BcMAM1显著上调,脂肪族硫苷的合成也显著增加,GBN的响应尤为强烈,说明脂肪族硫苷尤其是GBN参与了白菜对甜菜夜蛾幼虫的防御反应。然而,BcBCAT4、BcMAM1的表达水平与相应脂肪族硫苷的质量摩尔浓度存在一定差异,说明除了硫苷生物合成基因的直接影响外,可能还涉及其他机制来参与十字花科芸薹属作物次级代谢产物硫苷的生物合成和积累。此外,吲哚族的4OH、NEO、GBC在甜菜夜蛾幼虫取食过程中显著增加,说明吲哚族硫苷可能在白菜抵御甜菜夜蛾幼虫取食过程中发挥着重要的作用。KUMAR等[39]在研究害虫的质量与芥菜Brassica juncea中硫苷各组分的关系时,发现棉铃虫Helicoverpa armigera幼虫的增重与芥菜中GNA和GBN的含量呈负相关,而斜纹夜蛾Spodoptera litura幼虫的增重与GNA、GBN和黑介子硫苷酸钾(SIN)的含量呈负相关,进一步研究发现硫苷介导的芥菜对棉铃虫和斜纹夜蛾的抗性差异与BjuMYB28同源物在芥菜叶片中的差异表达相关。本研究中,甜菜夜蛾幼虫取食胁迫下,白菜4OH、NEO、GBC的增加与幼虫体内解毒酶GSTs活性及白菜硫苷合成相关基因BcSOT16呈正相关,说明BcSOT16活性的提高可能是甜菜夜蛾幼虫取食下白菜吲哚族硫苷合成增加的原因,进而提高了白菜对甜菜夜蛾的抗性。需进一步验证BcSOT16的生物学功能,以明确BcSOT16在白菜抵御甜菜夜蛾幼虫取食胁迫过程中所起到的重要作用。
在长期进化过程中,为抵御植食性昆虫的取食,植物形成了多种防御机制,如产生各种次级代谢物[40]。硫苷及其降解产物对昆虫具有阻食、影响其生长发育及繁殖,甚至毒杀的作用[11],然而,硫苷也能诱导昆虫体内细胞色素P450s和GSTs等主要解毒酶系基因的表达,增强昆虫对硫苷等植物次生物质的代谢能力[23-24, 41]。如草地贪夜蛾Spodoptera frugiperda幼虫的细胞色素P450s活性可以被吲哚族硫苷降解产物吲哚-3-甲醇所诱导[42];食用含硫苷、异硫氰酸酯的饲料或直接取食十字花科植物时,桃蚜体内GSTs活性会被诱导增加[24];与对照相比,取食含硫苷的桃蚜,食蚜蝇GSTs活性亦有所提高[43]。本研究中甜菜夜蛾幼虫取食白菜后,其体内GSTs活性不断增强,与白菜中总硫苷及吲哚类硫苷质量摩尔浓度增加趋势的一致性,表明了硫苷对甜菜夜蛾幼虫GSTs活性的诱导作用,也说明吲哚族硫苷可能在白菜抵御杂食性害虫甜菜夜蛾幼虫取食过程中发挥更为重要的作用。
-
甜菜夜蛾幼虫取食能上调白菜BcSOT16、BcBCAT4、BcMAM1等硫苷合成关键基因的表达,从而促进白菜硫苷质量摩尔浓度的增加,提高白菜对甜菜夜蛾幼虫取食胁迫的抗性,吲哚族的4OH、NEO、GBC和脂肪族的GBN的反应尤为强烈,但硫苷质量摩尔浓度的升高又会诱导甜菜夜蛾幼虫体内GSTs活性的增强,提高甜菜夜蛾幼虫对硫苷的解毒能力。
Molecular mechanism of glucosinolate-mediated Brassica campestris ssp. chinensis against feeding stress of Spodoptera exigua larvae
doi: 10.11833/j.issn.2095-0756.20220172
- Received Date: 2022-02-23
- Accepted Date: 2022-09-14
- Rev Recd Date: 2022-09-05
- Available Online: 2023-01-18
- Publish Date: 2023-01-17
-
Key words:
- glucosinolates /
- Brassica campestris ssp. chinensis /
- Spodoptera exigua /
- gene expression /
- glutathione S-transferase
Abstract:
| Citation: | HAO Jiaojiao, MA Yonghua, LU Yanchi, et al. Molecular mechanism of glucosinolate-mediated Brassica campestris ssp. chinensis against feeding stress of Spodoptera exigua larvae[J]. Journal of Zhejiang A&F University, 2023, 40(1): 81-88. DOI: 10.11833/j.issn.2095-0756.20220172 |












 DownLoad:
DownLoad: