-
磷是森林生态系统中重要的限制营养元素,参与生物体的各种代谢,其有效性在植物净初级生产力和微生物活性中起着重要作用[1]。为研究土壤磷的有效性,一般将磷分为无机磷和有机磷两大类,利用不同的化学提取液对土壤磷进行分级[2]。植物根系结合微生物利用一系列机制增加土壤磷的有效性[3],传统的化学分级方法没有充分反映植物根系和微生物介导磷转化过程。基于此,DELUCA等[4]提出了基于生物有效性的磷分级方法(biologically based phosphorus method,BBP法),定义了4种形式的生物可利用土壤磷组分,分别为用氯化钙提取的根系拦截和扩散的可溶性无机磷(CaCl2-P),用柠檬酸盐提取的吸附在黏土颗粒上或弱结合在无机沉淀物上的活性无机磷(Citrate-P),用酶提取被酸性磷酸酶和植酸酶水解的有机磷(Enzyme-P),用盐酸提取被植物和微生物产生的具有可溶性的活性无机磷质子(HCl-P)。该方法考虑到土壤根际与微生物分泌活化的磷表征磷组分[5],有助于深入理解生态系统内驱动磷转化的生物学机制。
由于人为排放的活性氮含量增加,大气氮沉降加剧[6]。中国亚热带地区是氮沉降最严重的地区之一,在过去30 a里氮沉降量的增幅约60%[7-8]。相关研究表明:长期高强度氮输入会改变土壤微生物生物量和活性,增加土壤酸性磷酸酶活性,从而影响土壤磷循环过程[9-11]。因此,在氮沉降背景下维持有效磷的供应来满足较高森林生产力已成为重要问题。毛竹Phyllostachys edulis是东亚和东南亚地区广泛栽植的竹种[12],在中国面积为467.78万 hm2,占全国竹林面积的72.96%,是重要的森林资源,在缓解气候变化方面具有重要的潜力[7, 13-14]。毛竹是无性繁殖植物,由复杂的地下根鞭上的笋芽发育而成[15],地下根系生物量占地上生物量的30%~50%,远高于其他类型森林生态系统[7, 16]。因此,毛竹林土壤磷循环尤其是生物有效磷组分极有可能与其他森林不同。毛竹主要分布在中国亚热带地区,其氮沉降量已经达30 kg·hm−2·a−1 [17]。LI等[11]研究发现:氮添加增加了毛竹林凋落物量、磷归还量、土壤酸化、土壤微生物生物量磷和有效磷,但降低了叶片磷养分吸收率和丛枝菌根真菌(arbuscular mycorrhizal fungi, AMF)侵染率[18],而氮沉降对毛竹土壤磷组分的研究还未见报道,制约了对大气氮沉降增强背景下毛竹林生产力响应机制的认识。本研究拟在不同氮添加强度处理下,分析毛竹林土壤不同深度磷组分的含量及其影响因素,量化各组分对土壤有效磷的贡献程度,为改进毛竹林经营管理措施、提升毛竹林生产力提供科学依据。
-
样地位于浙江省杭州市临安区青山湖街道。该地区属于亚热带季风气候,年均气温为15.6 ℃,年均降水量为1 420 mm,年均日照时数为1 847 h,年均无霜期约230 d,地形地貌为低山丘陵,海拔为100~300 m,土壤类型属于黄土壤[19]。
-
2012年11月在样地内设立了12个20 m×20 m的典型样地,并在样地间设置了20 m的缓冲隔离区以避免附近地块的干扰。以当地平均大气氮沉降速率约30 kg·hm−2·a−2和年平均铵态氮/硝态氮比值(NH4 +/NO3 −)1.28为依据,以硝酸铵(NH4NO3)为氮源[17],设置了4个氮沉降处理水平,分别为对照(0 kg·hm−2·a−1,N0)、低氮(30 kg·hm−2·a−1,N30)、中氮(60 kg·hm−2·a−1,N60)和高氮(90 kg·hm−2·a−1,N90),每个处理重复3次。从2013年1月开始每月初配置对应样地所需质量的NH4NO3溶于10 L水中,使用电动喷雾器将配好的溶液均匀地喷洒在每个样地上,1 a进行12次等量的喷洒,同时在对照组内采用同样的方法喷洒10 L水,避免加水对试验的影响。受新冠病毒疫情影响,样地从2020年1月起停止喷氮。
-
2020年8月,在每个样地内随机选取5个点,去除土壤上层凋落物,使用土钻对各处理样地的土壤分为表层(0~20 cm)和深层(20~40 cm)进行采样,将各土层的土样均匀混合,装入无菌袋后放入4 ℃便携式恒温箱中带回实验室。带回后立即对土壤进行预处理,剔除其中的石块、根系等杂物后,过2 mm土筛,将土样分为3份,分别作自然风干及储存于4 ℃和−20 ℃冰箱内低温保存处理。
土壤pH采用PHS-3C酸度计测定,土壤有机碳和土壤全氮通过碳氮元素分析仪测定,土壤全磷采用高氯酸-硫酸(HClO4-H2SO4)法测定,土壤有效磷采用0.5 mol·L−1 碳酸氢钠(NaHCO3)浸提,钼蓝比色法测定。土壤微生物生物量磷采用0.5 mol·L−1 NaHCO3作为土壤浸提剂的氯仿熏蒸浸提法[20]。土壤酸性磷酸酶活性采用对硝基苯磷酸二钠比色法测定[21]。
基于生物有效性的土壤磷分级测定采用DELUCA等[4]提出的BBP方法。分别称取0.5 g土壤加入4个15 mL离心管,分别平行加10 mL提取液,其中0.01 mol·L−1 CaCl2提取液用于提取CaCl2-P,10 mmol·L−1柠檬酸提取液用于提取Citrate-P,0.02×16.67 μkat·L−1酶提取液用于提取Enzyme-P,1 mol·L−1盐酸提取液用于提取HCl-P,震荡3 h,离心提取上清液。所有提取液稀释并采用孔雀石绿法测定磷质量分数[22]。
-
采用可重复双因素方差分析(two-way ANOVA)探讨氮添加和土壤深度及两者的交互作用对磷组分和土壤理化性质的影响。采用单因素方差分析(one-way ANOVA)和最小显著差异法(LSD)探讨不同氮添加处理间土壤理化性质和磷组分差异,利用Pearson相关系数分析土壤磷组分与土壤理化性质的相关性。采用SPSS 25.0软件完成数据分析,所有数据为平均值±标准差。
-
可重复双因素方差分析(表1)表明:除总磷外,氮添加、土壤深度及两者复合作用均显著影响了土壤理化性质(P<0.05)。由表2可知:与对照组相比,表层土壤中,氮添加显著降低了pH (P<0.05),降幅为5.2%~6.2%,显著增加了表层土壤中的全磷、有效磷和微生物生物量磷质量分数及酸性磷酸酶活性(P<0.05),增幅分别为14.3%~23.8%、62.4%~197.8%、100.1%~229.1%和47.9%~79.2%;添加低氮和高氮显著增加了表层土壤有机碳质量分数(P<0.05),增幅分别为27.2%和4.7%;添加中氮显著降低了表层土壤有机碳质量分数 (P<0.05),降幅为3.9%。在深层土壤中,氮添加显著降低了土壤pH和有机碳质量分数(P<0.05),降幅分别为6.2%~11.6%和9.3%~33.3%;显著增加了土壤全磷、有效磷和微生物生物量磷质量分数及酸性磷酸酶活性 (P<0.05),增幅分别为26.5%~40.8%、36.4%~175.5%、34.3%~59.3%、23.7%~76.3%。在对照组和氮添加处理下,深层土壤中的pH显著高于表层土壤(P<0.05),高3.5%~10.7%,而有机碳质量分数、有效磷质量分数和酸性磷酸酶活性显著低于表层土壤(P<0.05),分别低49.6%~185.3%、320.9%~819.3%、26.3%~46.8%。深层土壤全磷质量分数在低氮和中氮添加下分别显著提高了32.7%和29.2% (P<0.05),微生物生物量磷质量分数在中氮添加下显著增加了20.2% (P<0.05),在高氮添加下显著降低了23.7% (P<0.05)。
项目 氮添加 土壤深度 氮添加×土壤深度 F η2 F η2 F η2 pH 79.76** 0.93 253.45** 0.94 12.09** 0.69 SOC 82.37** 0.94 603.42** 0.99 41.48** 0.89 TN 105.50** 0.95 213.81** 0.99 7.46* 0.58 TP 81.43** 0.94 68.09** 0.81 0.38 0.07 AP 87.08** 0.94 101.26** 0.95 82.86** 0.94 MBP 452.98** 0.98 60.66** 0.79 182.38** 0.97 ACP 512.74** 0.99 124.97** 0.94 22.77** 0.63 CaCl2-P 24.50** 0.82 8.26* 0.34 1.59 0.23 Citrate-P 118.65** 0.93 65.89** 0.92 39.42** 0.84 Enzyme-P 30.23** 0.65 8.13* 0.60 9.02* 0.63 HCl-P 574.53** 0.99 86.86** 0.94 81.68** 0.94 说明:pH指酸碱度;SOC指有机碳;TN指总氮;TP指总磷;AP指有效磷;MBP指土壤微生物生物量磷;ACP指酸性磷酸酶活性; η2指方差分析中的效应量。*P<0.05;**P<0.01 Table 1. Two-way ANOVA about the effects of N addition, soil depth and their interactions on the concentrations of P fraction content and soil physicochemical properties
土壤深度/cm 处理 pH SOC/(g·kg−1) TN/(g·kg−1) TP/(g·kg−1) AP/(mg·kg−1) MBP/(mg· kg−1) ACP/
(μmol·g−1·h−1)0~20 N0 4.20±0.07 aB 24.31±0.29 cA 2.29±0.09 aA 0.42±0.01 cA 4.63±0.09 dA 7.26±0.31 cB 0.48±0.02 dA N30 3.98±0.03 bB 30.93±0.16 aA 1.80±0.04 cA 0.52±0.01 aB 13.79±0.04 aA 14.53±0.16 bA 0.71±0.02 cA N60 3.97±0.06 bA 23.35±0.08 dA 2.10±0.01 bA 0.48±0.02 bB 7.52±0.03 cA 14.52±0.17 bB 0.79±0.01 bA N90 3.94±0.03 bB 25.45±0.50 bA 2.08±0.08 bA 0.52±0.01 aA 8.87±0.09 bA 23.89±0.67 aA 0.86±0.02 aA 20~40 N0 4.65±0.03 aA 16.25±0.05 aB 1.42±0.02 aB 0.49±0.02 cA 1.10±0.05 dB 11.44±0.34 dA 0.38±0.01 dB N30 4.36±0.05 bA 10.84±0.08 dB 0.96±0.01 dB 0.69±0.01 aA 1.50±0.05 cB 15.36±0.30 cA 0.47±0.04 cB N60 4.11±0.06 cA 14.74±0.31 bB 1.25±0.03 bB 0.62±0.01 bA 1.93±0.01 bB 17.45±0.50 bA 0.55±0.01 bB N90 4.21±0.03 cA 12.33±0.10 cB 1.01±0.01 cB 0.48±0.01 cA 3.03±0.04 aB 18.22±0.25 aB 0.67±0.11 aB 说明:不同小写字母表示在同一土壤深度处理下不同氮添加处理间差异显著(P<0.05);不同大写字母表示同一氮添加处理下不同土 壤深度间差异显著(P<0.05) Table 2. Soil physical and chemical properties of topsoil and subsoil under N addition treatments
-
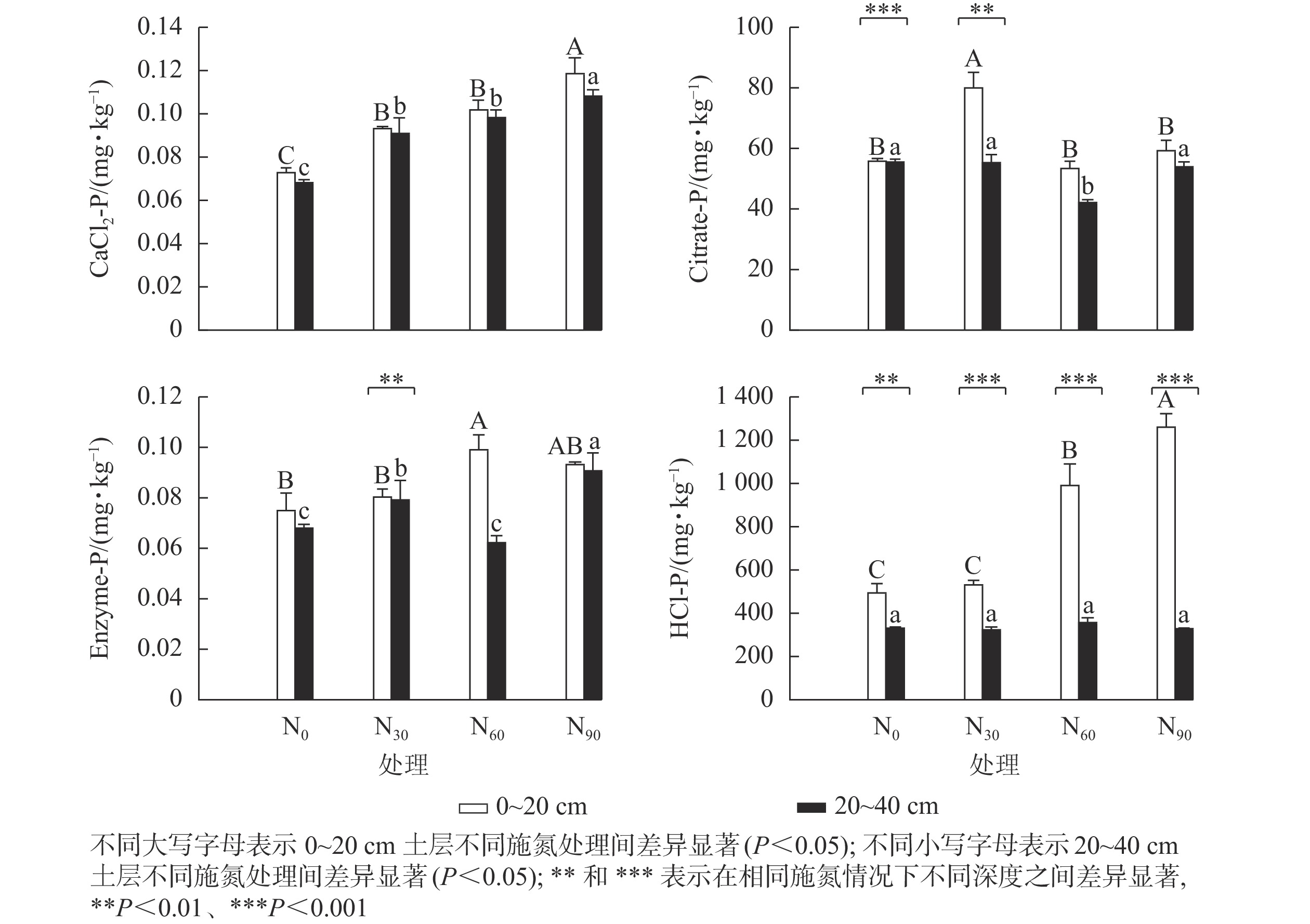
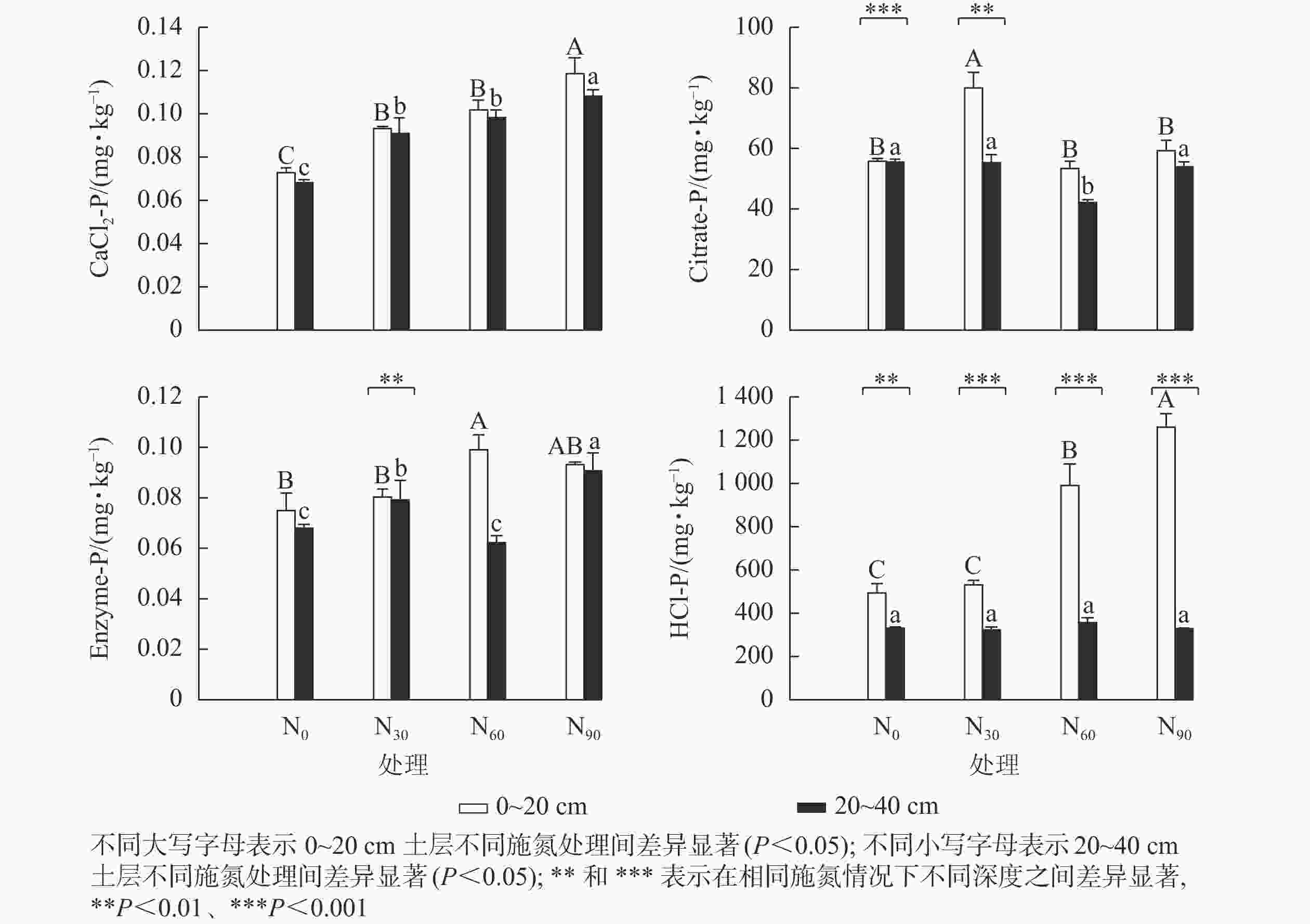
可重复双因素方差分析(表1)表明:氮添加和土壤深度及其复合作用,除CaCl2-P外,对其他几种生物有效性磷组分质量分数均具有显著影响(P<0.05)。图1显示:氮添加显著增加了基于生物有效性的土壤磷组分质量分数(P<0.05)。在表层土壤中,与对照组相比,低氮显著增加了CaCl2-P和Citrate-P质量分数(P<0.05),增幅分别为28.5%和43.5%;中氮显著增加了CaCl2-P、Enzyme-P和HCl-P质量分数(P<0.05),增幅分别为39.7%、32.4%和101.0%;高氮显著增加了CaCl2-P和HCl-P质量分数(P<0.05),增幅分别为63.3%和155.2%。在深层土壤中,与对照组相比,低氮显著增加了CaCl2-P和Enzyme-P质量分数(P<0.05),增幅分别为33.6%和33.6%;中氮显著增加了CaCl2-P质量分数(P<0.05),增幅为44.3%,而显著降低了Citrate-P质量分数(P<0.05),降幅为24.3%;高氮显著增加了CaCl2-P和Enzyme-P质量分数(P<0.05),增幅分别为58.6%和33.6%;氮添加对HCl-P质量分数无显著影响,HCl-P质量分数随土壤深度的增加显著降低。在中氮处理下,Citrate-P和Enzyme-P质量分数随土壤深度的增加显著降低。
-
相关性结果(表3)表明:表层土壤有效磷与各磷组分均呈显著正相关(P<0.05),其中有效磷质量分数与Citrate-P质量分数的相关性最高(r=0.696),与Enzyme-P (r=0.522)和HCl-P (r=0.417)次之,与CaCl2-P最低(r=0.375);深层土壤有效磷与Enzyme-P呈显著正相关(P<0.01,r=0.711)。
项目 土壤深度/cm CaCl2-P Citrate-P Enzyme-P HCl-P AP 0~20 0.375* 0.696** 0.522** 0.417* 20~40 0.286 −0.151 0.711** −0.493 说明:*P<0.05;**P<0.01 Table 3. Correlation coefficients between P components and available P in different soil depth
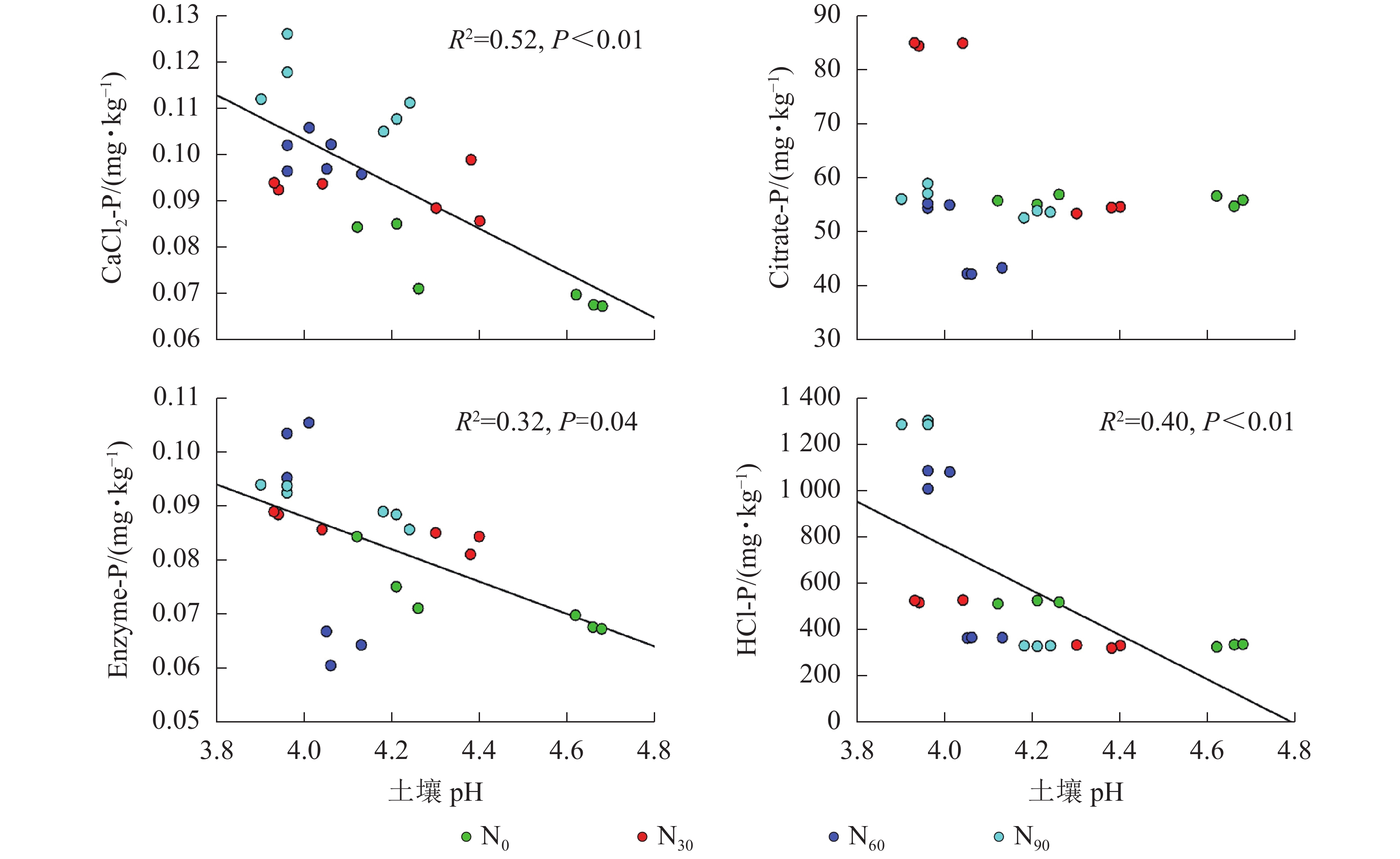
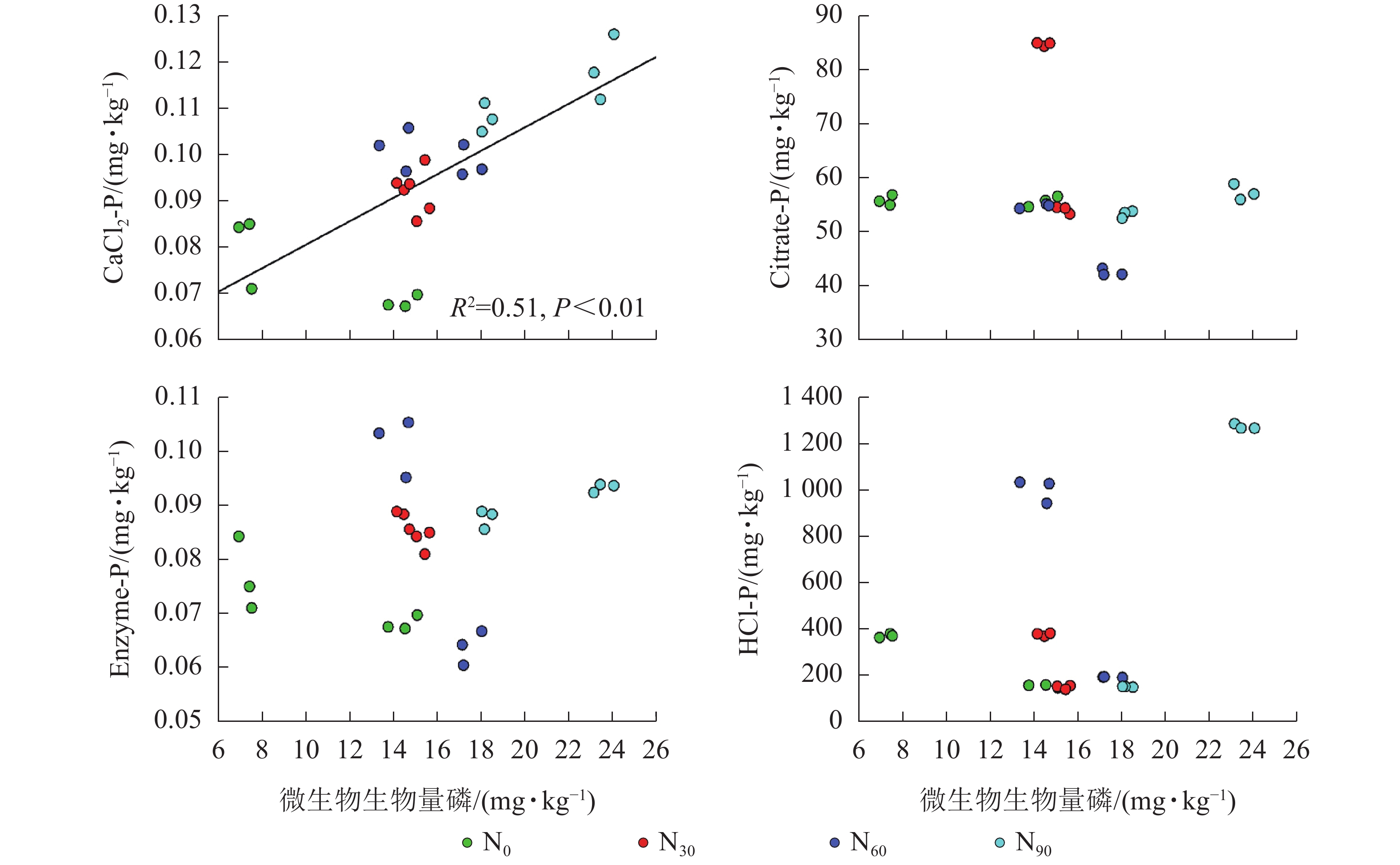
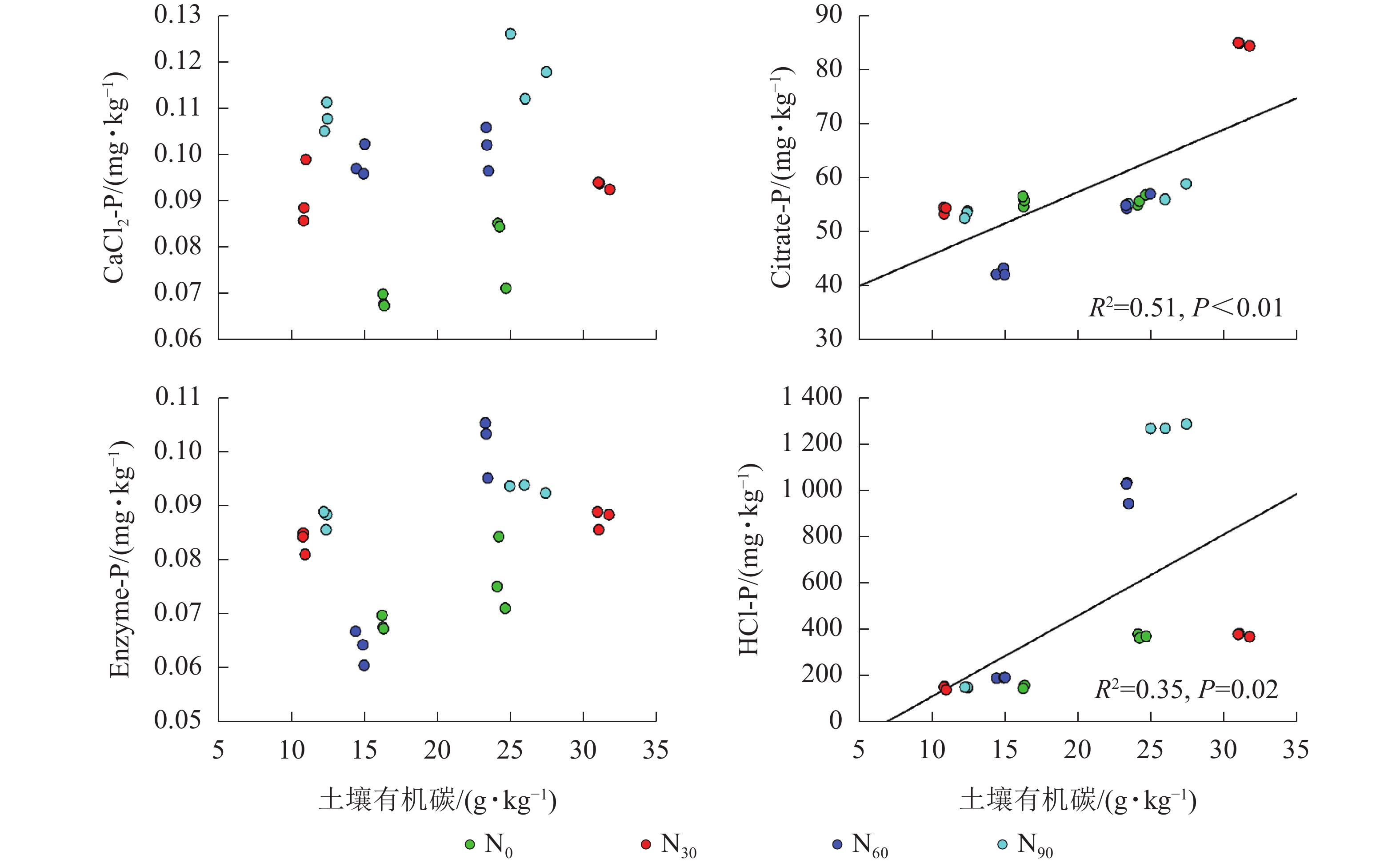
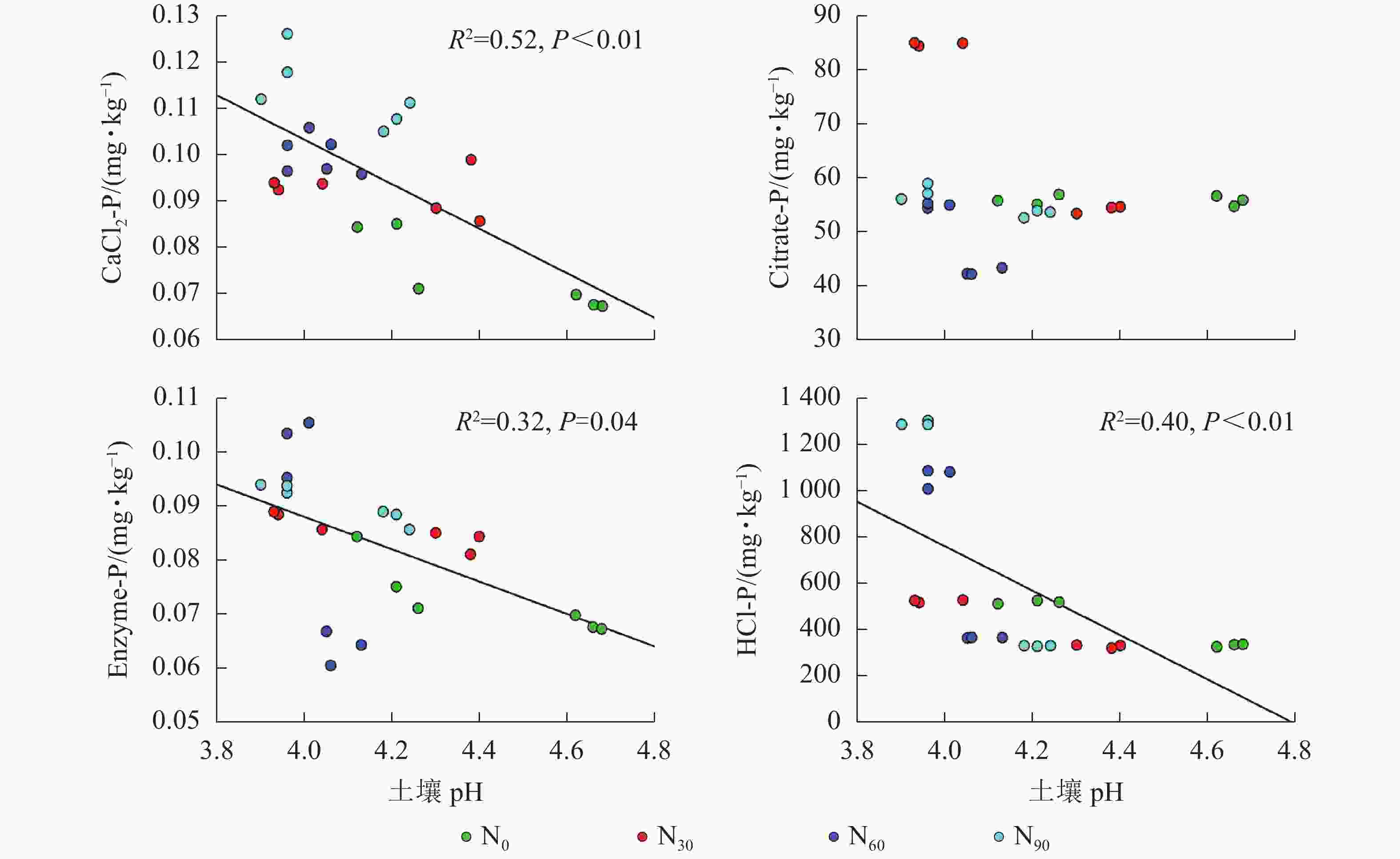
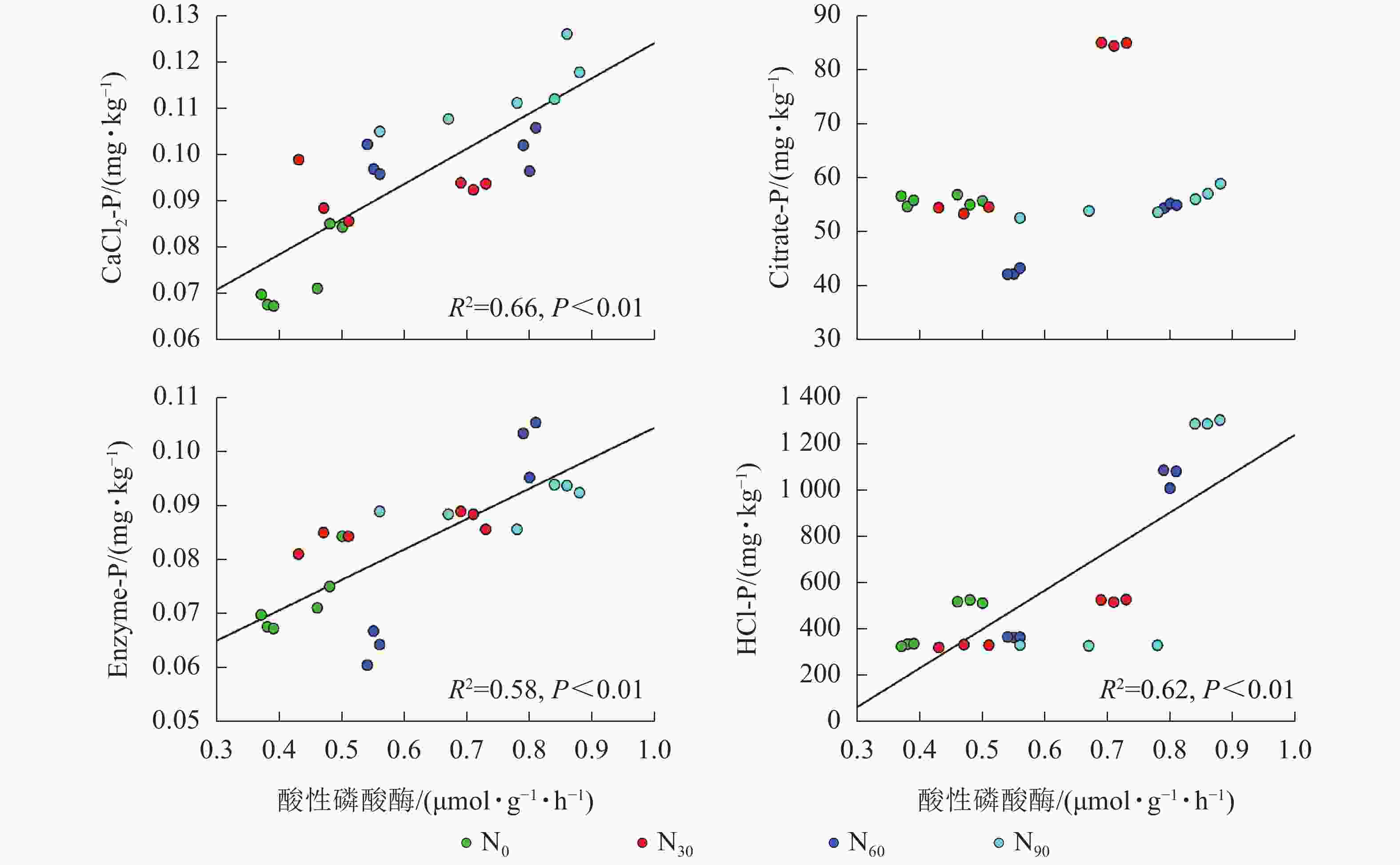
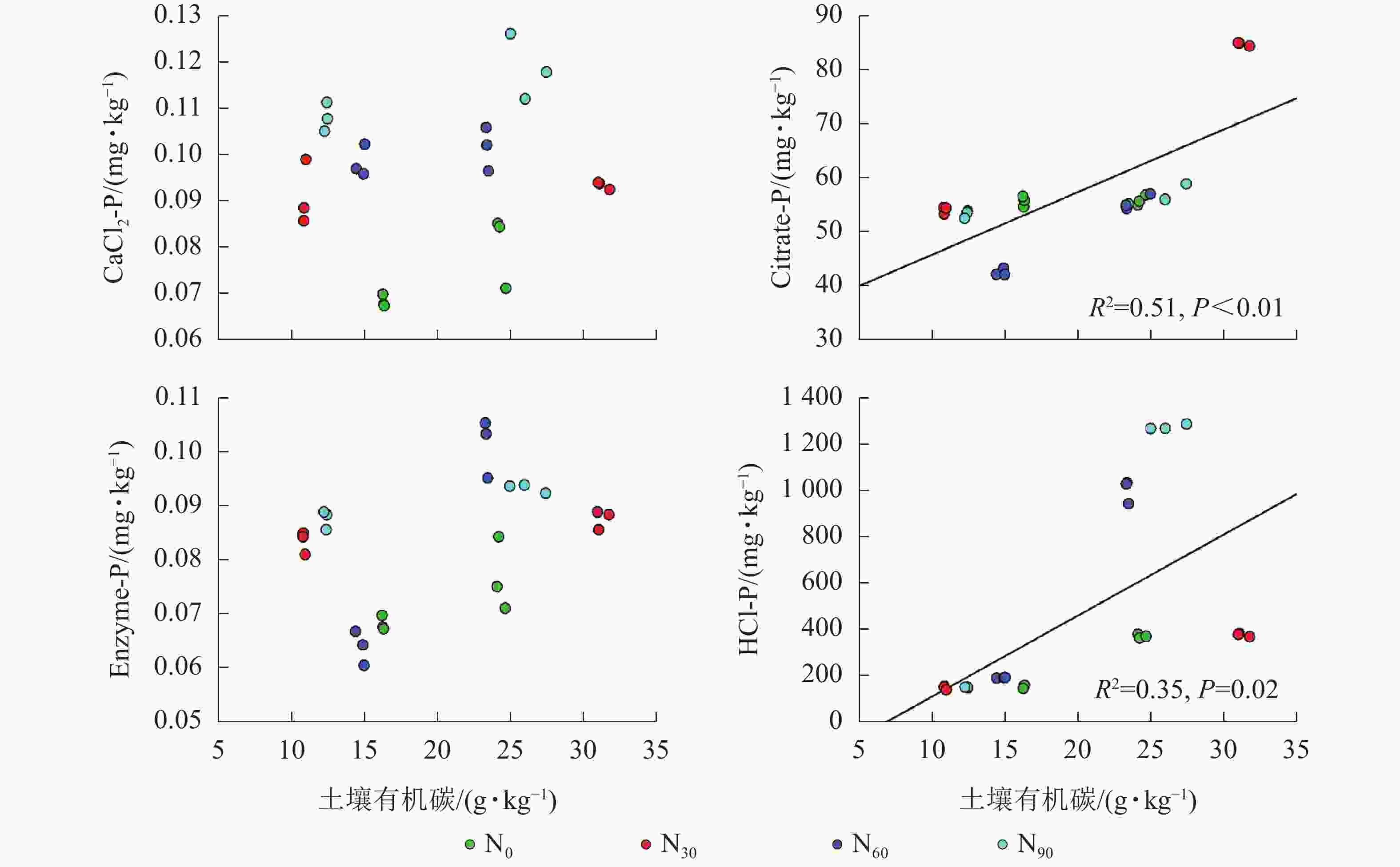
由图2~5可见:土壤pH与CaCl2-P(P<0.01)、Enzyme-P (P<0.05)和HCl-P (P<0.01)呈显著负相关,土壤酸性磷酸酶活性与CaCl2-P、Enzyme-P和HCl-P呈显著正相关(P<0.01),土壤微生物生物量磷与CaCl2-P呈显著正相关(P<0.01),土壤有机碳与Citrate-P (P<0.01)和HCl-P (P<0.05)呈显著正相关。土壤pH和酸性磷酸酶活性对CaCl2-P (P<0.01)的影响更为显著。土壤有机碳对Citrate-P (P<0.01)的影响更为显著。
-
本研究中,除深层土壤中HCl-P质量分数外,氮添加显著增加了各土层生物有效性磷质量分数。主要原因是氮添加导致了植物和土壤中氮磷摩尔比远小于陆地生态系统中的氮磷摩尔比[23]。在表层和深层土壤中,所有水平的氮添加显著增加了CaCl2-P质量分数,原因可能是氮添加增加了毛竹林根系生物量[7],增加了根系对无机磷的拦截,从而提高了土壤中CaCl2-P质量分数。在表层土壤中,Citrate-P质量分数仅在低氮处理下显著增加,而在中氮和高氮处理下无显著变化,原因是中、高氮处理下土壤中铵态氮和硝态氮离子含量显著提高,促使土壤中离子达到饱和状态[24],从而抑制了根系分泌的有机酸对磷的活化作用。中、高氮添加处理提高了Enzyme-P和HCl-P质量分数,这是由于中、高氮处理显著增加了酸性磷酸酶(ACP)活性,降低了土壤pH。相关性分析也表明:Enzyme-P和HCl-P质量分数与土壤pH呈显著负相关,而与ACP呈显著正相关,支持了上述观点。在深层土壤中,所有水平的氮添加均显著增加了Enzyme-P质量分数,但对HCl-P质量分数无显著影响,表明氮添加促进了深层土壤中的有机磷转化,而对中等无机磷转化无显著影响。在对照和氮添加处理下,表层土壤中各磷组分的质量分数高于深层土壤,尤其是HCl-P质量分数。这可能是因为毛竹林表层土壤中根系生物量和微生物量高于深层土壤,高的根系和微生物量和活性加剧了土壤磷的转化,从而增加了土壤生物有效磷质量分数。陈红等[25]研究发现:毛竹表层土壤(0~20 cm)的根系生物量是深层土壤(20~40 cm)的2.35倍,且毛竹土壤微生物生物量随土壤深度的增加而减少[26],支持了上述观点。因此表层土壤会提供更多的生物有效磷。
对照及其氮添加处理下的4种磷组分质量分数由高到低依次是HCl-P、Citrate-P、CaCl2-P、Enzyme-P,氮添加未改变4种磷组分质量分数分布模式,表明土壤生物有效性磷主要以无机形式存在,不会受氮添加的影响。表层土壤中有效磷与所有磷组分间均有显著的正相关关系,与Citrate-P的相关系数最大,与Enzyme-P的相关系数次之,表明毛竹林表层土壤中的有效磷主要来自于有机酸活化释放的无机磷。在农田生态系统中也发现Citrate-P的贡献最大[27]。深层土壤中有效磷仅与Enzyme-P呈显著正相关,表明毛竹林深层土壤有机磷库的酶矿化显著影响了有效磷,DODD等[28]研究发现:土壤有机磷库矿化直接转化为有效磷,影响了有效磷的水平。
-
土壤pH显著影响土壤磷组分,但影响程度和方向有所不同[29]。本研究发现:土壤pH与土壤磷组分质量分数呈显著负相关,这与DELUCA等[4]的大尺度研究结果相反。氮添加导致土壤pH显著降低,土壤pH降低会加速土壤有机磷的矿化,从而增加有效磷质量分数[30]。有研究表明:酸性土壤中磷的有效性与铁、铝参与的化学过程密切相关,土壤有效磷增加会和可交换铝离子(Al3+)和铁离子(Fe3+)结合形成稳定的磷[31]。短时间内由于土壤磷限制,这些可利用磷会被植物和土壤中微生物利用,但长期的氮添加可能使土壤中的Al3+和Fe3+吸附和固定作用降低[32],从而导致土壤生物有效性磷的质量分数降低。因此,需要加强土壤pH与土壤磷组分关系的研究。
微生物在土壤磷的转化过程中起着重要作用[33-34]。土壤微生物是土壤生态环境中最为重要且对环境变化极其敏感的生物活性因子[35]。施氮会影响土壤微生物活动,DENG等[36]研究表明:低水平的氮添加促进微生物生长,而当氮添加的量超过饱和点后会对微生物生长表现出抑制作用。本研究发现:氮添加显著增加了土壤微生物生物量磷,从而增加了土壤的有效磷,主要原因是氮肥的成分硝酸铵中存在NH4 +,且土壤pH降低,都有助于微生物分解无机磷。土壤微生物生物量磷与CaCl2-P质量分数呈显著正相关,表明土壤磷与微生物显著相关。土壤微生物的代谢和活性还与土壤有机碳密切相关[37]。氮添加加快了土壤表层凋落物分解,叶凋落物生物量与土壤有机碳呈负相关[38],从而导致土壤有机碳含量增加。土壤中有机碳含量的增加有助于形成更丰富活跃的土壤微生物群落,促进菌根的形成和无机活性质子的增加[39],从而增加生物有效性磷含量。土壤有机碳作为微生物活动能量的主要来源[40],它的增加还可以提高脲酶和磷酸酶等的活性[41]。土壤酸性磷酸酶是酸性土壤中参加磷矿化的重要酶类。酸性磷酸酶与CaCl2-P、Enzyme-P和HCl-P呈显著正相关。磷酸酶中的氮含量相对较高,在它们参与的反应过程中需要大量的氮,因此氮添加可以显著增加土壤酸性磷酸酶的产量和活性,从而提高磷的生物有效性[42-43],减轻磷限制,有利于毛竹林生产力的提升[7]。
Effect of nitrogen addition on soil phosphorus fractions in the Phyllostachys edulis plantation
doi: 10.11833/j.issn.2095-0756.20220236
- Received Date: 2022-03-18
- Rev Recd Date: 2022-04-25
- Available Online: 2022-07-20
- Publish Date: 2022-08-20
-
Key words:
- nitrogen input /
- bioavailable phosphorus /
- Phyllostachys edulis /
- soil microorganisms /
- acid phosphatase
Abstract:
| Citation: | WANG Yixiong, ZHANG Huafeng, LI Quan, et al. Effect of nitrogen addition on soil phosphorus fractions in the Phyllostachys edulis plantation[J]. Journal of Zhejiang A&F University, 2022, 39(4): 695-704. DOI: 10.11833/j.issn.2095-0756.20220236 |










 DownLoad:
DownLoad: