-
中国作为农业大国之一,具有丰富的秸秆资源。据统计,中国秸秆年生产量达8.65 亿t,但每年有1 亿t秸秆废置,造成资源浪费和环境污染[1]。秸秆主要成分为木质纤维素,其特殊的“纤维素-半纤维素-木质素”三维网络结构在自然状态下难以降解,严重限制了秸秆资源利用[2]。随着技术不断发展,目前已开发出多种秸秆处理方法,如高温热解、蒸汽爆破、酸碱处理等[3]。微生物降解法以成本低、耗能小、环境友好等优点受到广泛关注[4]。相较于细菌和放线菌,真菌能产生更多种类的酶,如纤维素酶、木聚糖酶、果胶酶、过氧化物酶、糖苷酶等来协同降解木质纤维素[5−7],成为当前研究热点。目前,已开发的秸秆降解真菌主要有黄孢原毛平革菌Phanerochaete chrysosporium、黑曲霉Aspergillus niger、草酸青霉Penicillium oxalicu、糙皮侧耳Pleurotus ostreatus等[8−11],但部分真菌仍存在降解周期长、生长条件严格、降解不稳定等缺点[12],难以实现产业化,因此,仍需进一步挖掘高效稳定的木质纤维素降解真菌。
白蚁是自然界中高效的木质纤维素分解者,其特殊的白蚁-共生菌降解系统可消化植物中74%~99%的纤维素和65%~87%的半纤维素[13],降解效率为瘤胃动物的3倍以上[14]。有研究表明:白蚁的共生真菌具有较强的木质纤维素降解能力,如王成盼等[15]研究发现:黑翅土白蚁Odontotermes formosanus菌圃共生真菌蚁巢伞Termitomyces在固体发酵条件下可降解木质食料中20.98%的纤维素、31.89%的半纤维素和11.68%的木质素。SIJINAMANOJ等[16]从胖身土白蚁O. obesus菌圃和肠道中分离得到红绶曲霉A. nomius和哈茨木霉Trichoderma harzianum,分别使不同农业废弃物纤维素降低10.83%~15.53%和8.50%~36.30%。
为进一步挖掘白蚁共生真菌在秸秆资源利用上的应用潜力,本研究从黑翅土白蚁肠道中分离筛选木质纤维素降解真菌,挖掘高效降解水稻Oryza sativa秸秆的真菌菌种资源,为秸秆生物降解产业化提供理论基础。
-
供试黑翅土白蚁蚁巢采自福建省三明市大田县,26 ℃避光饲养。供试水稻秸秆收集自江苏省连云港市,烘干粉碎后过100目筛,常温保存。
-
羧甲基纤维素钠(CMC-Na)培养基:CMC-Na 1.000 g、硝酸钠 0.200 g、氯化铵 0.200 g、磷酸二氢钾 0.100 g、磷酸氢二钾 0.100 g、蛋白胨 0.100 g、七水硫酸镁 0.050 g、氨苄青霉素 0.005 g、卡那霉素 0.005 g、琼脂 4.000 g、蒸馏水 100 mL,pH 7。
水稻秸秆培养基:水稻秸秆粉(100目) 2.000 g、硝酸钠 0.200 g、氯化铵 0.200 g、蛋白胨 0.200 g、磷酸二氢钾 0.100 g、磷酸氢二钾 0.100 g、七水硫酸镁 0.050 g、蒸馏水 100 mL,pH 7。
-
肠道匀浆制备:取黑翅土白蚁成熟工蚁25只,在无菌超净台中用体积分数为75%的乙醇浸泡消毒5 min,无菌水清洗3次后用解剖针压住腹部末端,拉出白蚁肠道,于5 mL质量分数为0.9%的无菌生理盐水中研磨成匀浆。
分离:肠道匀浆液稀释100倍后,取100 µL均匀涂布于CMC-Na平板上,37 ℃避光培养至单菌落,长成后划线分离,重复3次后获纯培养菌株。
筛选:将直径5 mm菌饼接种于CMC-Na平板,37 ℃避光培养5 d后加入20 mL 1 g·L−1的刚果红溶液,染色1 h弃去染色液,加入1 mol·L−1的氯化钠溶液20 mL脱色30 min,观察菌株是否产生透明圈。
-
将纯化后菌株送至浙江尚亚生物技术有限公司,提取分离真菌DNA后,选取通用引物ITS1与ITS4扩增真菌ITS序列,将扩增产物连接至pTOPO-T载体并转入感受态细胞,使用M13F/13R引物扩增载体序列,测序后通过BLAST在线比对菌种,同时于GenBank登记序列号。
-
取直径5 mm菌饼接种于100 mL CMC-Na液体培养基(配方同CMC-Na培养基,不加琼脂),于27 ℃、180 r·min−1的摇床内避光培养9 d后,取2 mL液体培养基,4 ℃ 8 000 r·min−1条件下离心5 min,上清液即为粗酶液。
-
取200 μL粗酶液(对照组取无菌CMC-Na液体培养基),加入800 μL 质量分数为1% CMC-Na溶液,50 ℃反应60 min后加入1 mL 3,5-二硝基水杨酸(DNS)溶液,沸水浴反应10 min,冷却后稀释至5 mL,于540 nm波长下测定吸光度值,计算酶活性。
-
取200 μL粗酶液(对照组取无菌CMC-Na液体培养基),加入800 μL 质量分数为1%微晶纤维素溶液,50 ℃反应60 min后加入1 mL 3,5-二硝基水杨酸(DNS)溶液,沸水浴反应10 min,冷却后稀释至5 mL,于540 nm波长下测定吸光度值,计算酶活性。
-
取100 μL粗酶液(对照组取无菌CMC-Na液体培养基),加入200 μL 10 mmol·L−1 对硝基苯基-β-D-吡喃葡萄糖(p-NPG)溶液,50 ℃反应30 min后加入2 mL 1 mol·L−1 碳酸钠溶液终止反应并显色,于400 nm波长下测定吸光度,计算酶活性。
-
本研究酶活性单位定义:在50 ℃,pH 7条件下,1 L酶液在1 s内水解底物生成1 μmol还原产物所需的酶量为1个酶活性单位(μkat·L−1)。
-
将CMC-Na平板分为2个半圆区域,分别在2个半圆区域内接种直径为0.5 cm的不同真菌菌饼,37 ℃黑暗条件下对峙培养7~15 d,观察真菌间拮抗反应。
-
将直径0.5 cm真菌菌饼(双菌组合各1/2,加入直径为0.5 cm菌饼)接入灭菌后水稻秸秆培养基(秸秆干质量记为m),37 ℃避光培养20 d,使用20目筛分离真菌菌丝和秸秆,秸秆80 ℃烘干12 h后称干质量(记为md),计算干物质降解率(Rdm):Rdm= (m−md)/m×100%。
-
木质纤维素组分质量分数测定参考美国国家可再生能源实验室(NREL)方法[17],并有所简化:取0.2 g干燥植物材料(记为m0),加入3 mL体积分数为72%的硫酸,摇匀,30 ℃水浴1 h。稀释酸体积分数至4%(体积记为v),转移至聚四氟乙烯耐压瓶中,于烘箱中121 ℃酸解2 h。使用砂芯漏斗抽滤,滤纸采用已烘干并称量的无灰定量滤纸(记为m1)。滤渣连同滤纸转移至陶瓷坩埚,80 ℃过夜烘干后称量(记为m2),随后于马弗炉中550 ℃煅烧4 h,冷却后称量(记为m3)。滤液以质量分数为4%的氢氧化钠中和至中性后(稀释倍数记为k),用葡萄糖试剂盒测定葡萄糖质量分数(记为Cglu),用DNS法测定还原糖质量分数(记为Crs),计算纤维素、半纤维素和木质素质量分数(分别记为Ccel、Chem和Clig),最后根据降解前组分质量分数(记为C)和降解后组分质量分数(记为Cd)计算组分降解率(记为Rlc)。计算公式如下:Ccel = Cglu×v×k×0.90/m0×100%;Chem = (Crs−Cglu)×v×k×0.88/m0×100%;Clig = (m2−m1−m3)/m0×100%;Rlc = [1−(m×C)/(md×Cd)]×100%。其中:0.90为纤维素单糖转化系数,0.88为半纤维素单糖转化系数。
-
取降解前后水稻秸秆样品,烘干后研磨成粉末,使用傅立叶红外光谱仪(Thermo Scientific NICOLET iS50FT-IR)分析。分辨率4 cm−1,扫描次数32次,扫描波长500~4 000 cm−1。
-
取降解前后水稻秸秆样品,烘干后研磨成粉末,使用X射线衍射仪(Bruker D8 Advance)分析。扫描范围5°~90°,扫描速度5°·min−1,计算纤维素结晶度(D) = (I002−Iam)/I002×100%。其中:I002表示2θ = 22.5°衍射峰最大值;Iam表示2θ = 18.0°衍射峰最小值。
-
取降解前后水稻秸秆样品,烘干后研磨成粉末后用扫描电镜(Hitachi SU8010)观察。加速电压:3.0 kV;放大倍数:1 000倍。
-
使用SPSS 29对不同真菌的纤维素酶活性和水稻秸秆降解率进行方差齐性检验与显著性分析。
-
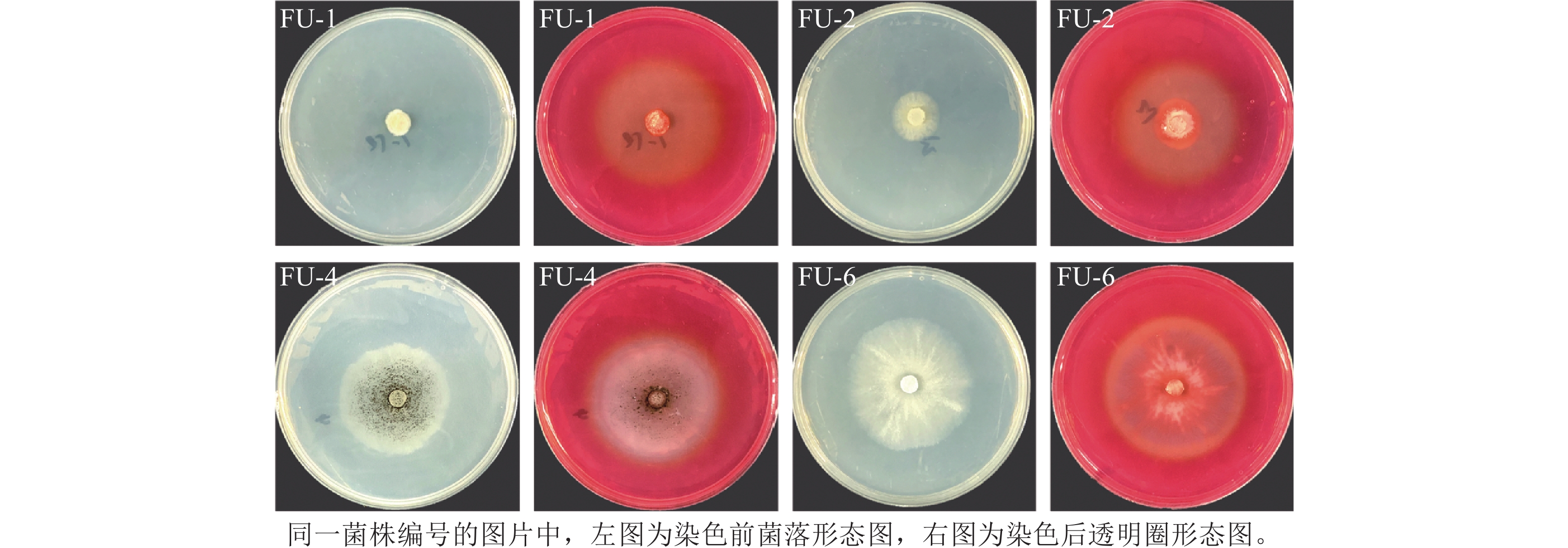
本研究共从黑翅土白蚁肠道中分离出6株真菌,分别记为FU-1~FU-6。经刚果红染色后筛选出FU-1、FU-2、FU-4和FU-6共4株产生明显透明圈的真菌,其菌落形态与透明圈形态如图1所示,其中产生透明圈初步表明其能将纤维素降解为多聚糖类物质,具有潜在的木质纤维素降解活性[18]。
-
对4株具备木质纤维素降解活性的真菌进行分子生物学鉴定,其中FU-1、FU-2属于篮状菌属Talaromyces,分别鉴定为安拉阿巴德篮状菌T. allahabadensis和刺孢篮状菌T. aculeatus。FU-4属于曲霉属Aspergillus,鉴定为黑曲霉A. niger。FU-6属于多年卧孔菌属Perenniporia,鉴定为灰孔多年卧孔菌P. tephropora(表1)。
菌株编号 GenBank
登记号相似菌种
(GenBank登记号)相似度/
%FU-1 OQ804643 Talaromyces allahabadensis
(MH727608)100.00 FU-2 OQ804660 T. aculeatus (HQ392496) 100.00 FU-4 OQ804669 Aspergillus niger
(MG228418)100.00 FU-6 OQ804677 Perenniporia tephropora
(MW077095)99.84 Table 1. Identification results of four fungal strains
-
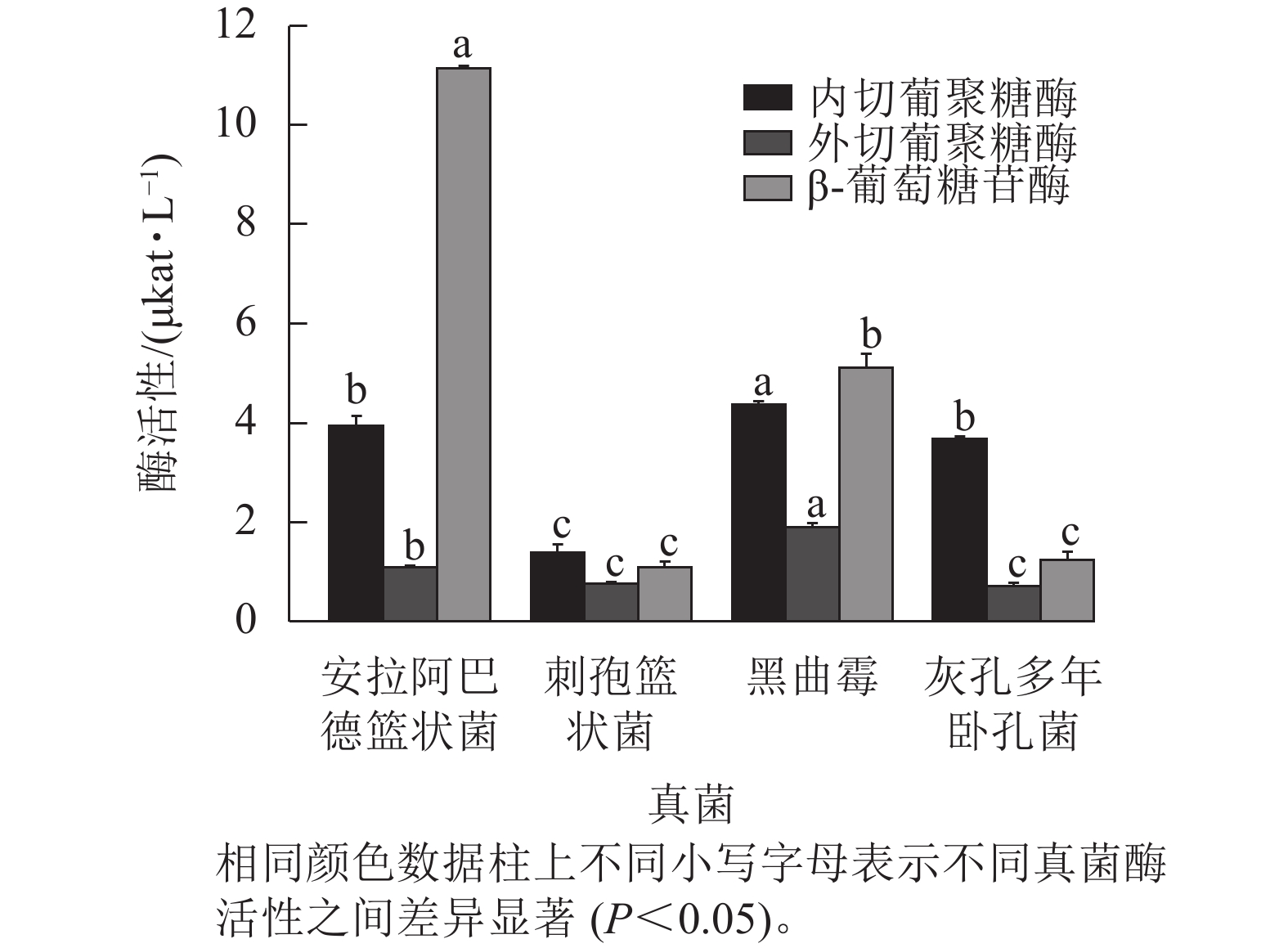
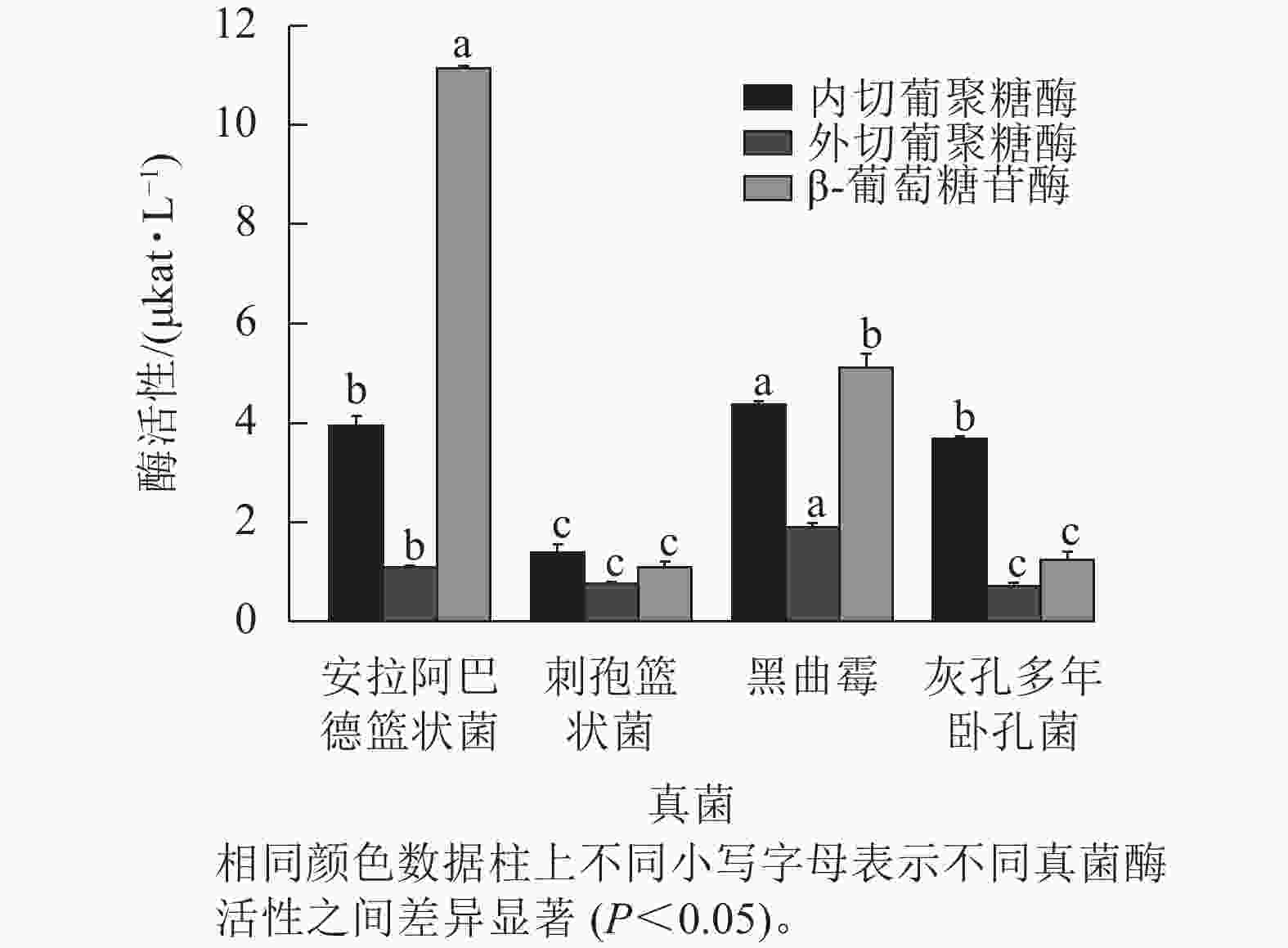
图2结果表明:黑曲霉的内切葡聚糖酶和外切葡聚糖酶活性显著高于其他真菌(P<0.05),分别达4.361和1.893 μkat·L−1,表明其具有较强的纤维素链切割和末端水解能力。安拉阿巴德篮状菌的β-葡萄糖苷酶活性显著高于其他真菌(P<0.05),达11.133 μkat·L−1,说明具有较强的纤维二糖水解能力。
-
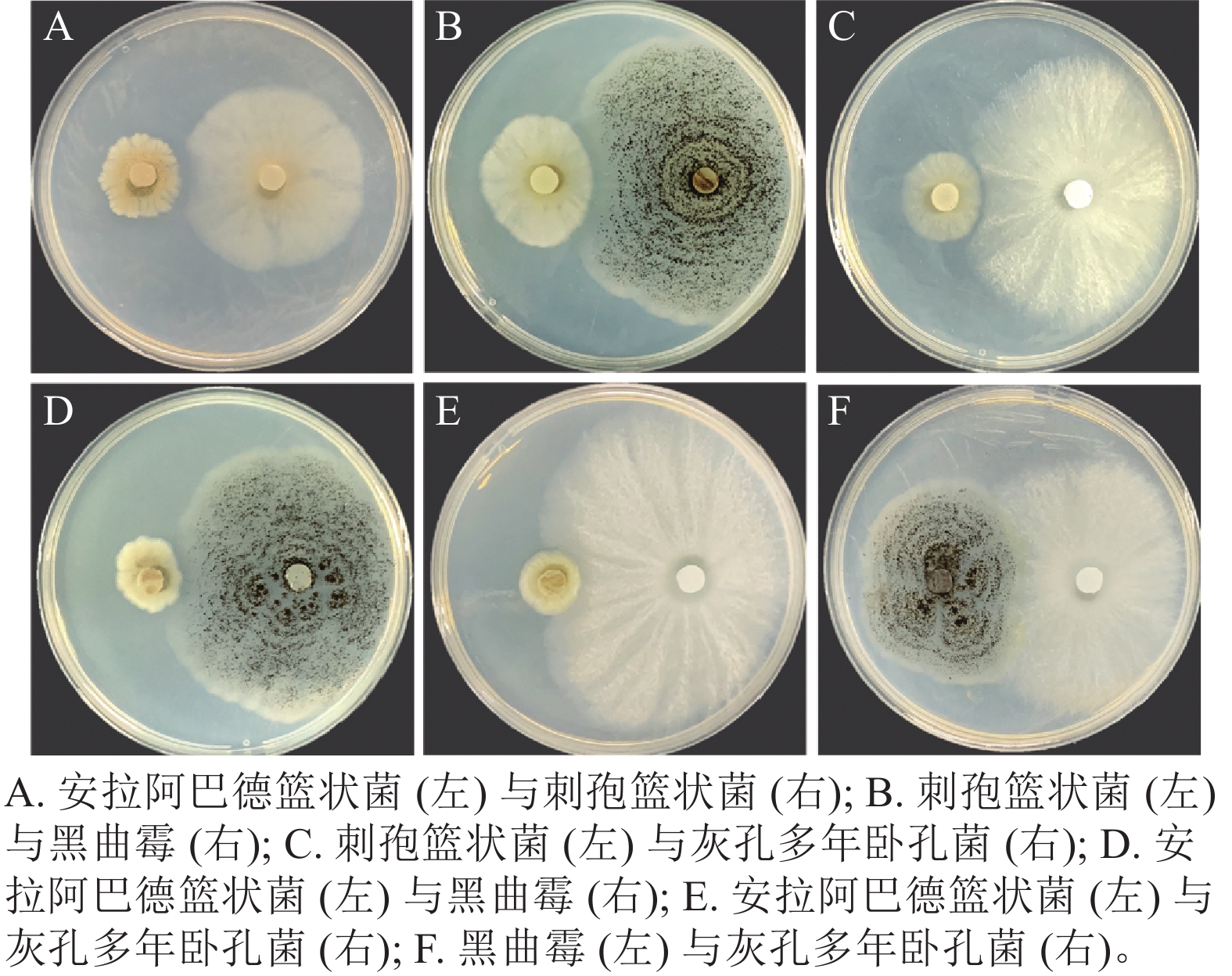
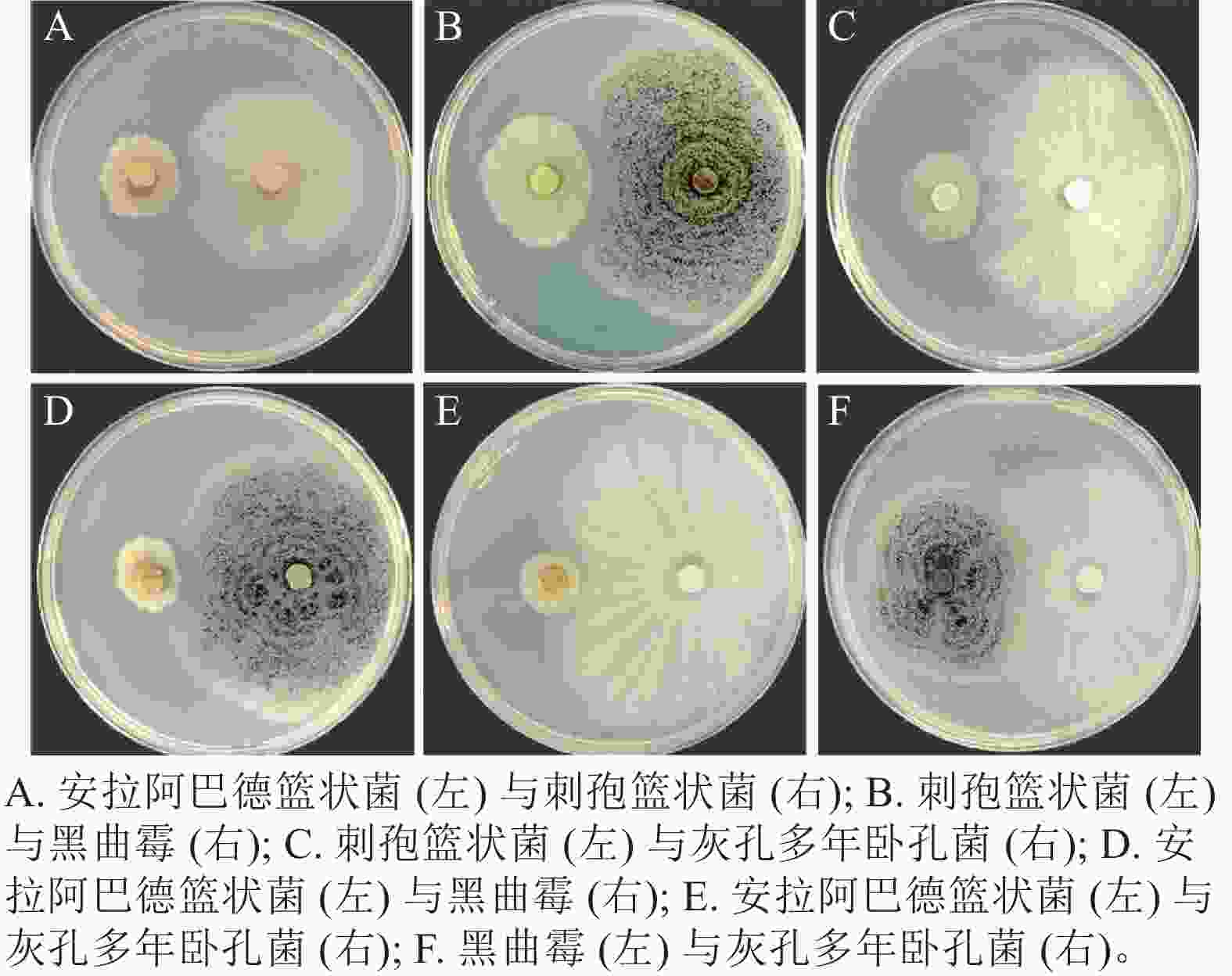
将筛选得到的4株真菌两两组合对峙,发现安拉阿巴德篮状菌与刺孢篮状菌(图3A)、刺孢篮状菌与黑曲霉(图3B)和刺孢篮状菌与灰孔多年卧孔菌(图3C)菌落之间产生抑菌圈,表现出相互拮抗。而安拉阿巴德篮状菌与黑曲霉(图3D)、安拉阿巴德篮状菌与灰孔多年卧孔菌(图3E)和黑曲霉与灰孔多年卧孔菌(图3F)菌落之间可接触生长,具备共生协作降解木质纤维素的潜能。
-
单独降解时,4株真菌均表现出一定降解能力(表2),其中刺孢篮状菌和黑曲霉具有显著较高的纤维素降解率,分别达38.49%和40.72%,灰孔多年卧孔菌具有极高的半纤维素降解率,达59.77%,同时这3种真菌的木质素降解率也达到最高水平。组合降解时,部分真菌组合表现出更强的降解能力,其中当黑曲霉与灰孔多年卧孔菌“强强联合”后,其干物质降解率和纤维素降解率进一步提高,达38.27%和62.59%,同时半纤维素降解率仍保持较高水平,达51.75%。尽管其木质素降解率有所降低,但综合来看,该真菌组合表现出最强的水稻秸秆降解能力,具有潜在的开发价值。
处理组 干物质降解率/% 纤维素降解率/% 半纤维素降解率/% 木质素降解率/% 安拉阿巴德篮状菌 26.38±0.33 e 27.86±0.49 e 19.44±2.05 d 13.06±2.56 c 刺孢篮状菌 33.79±1.16 bc 38.49±0.93 c 21.75±2.20 d 28.96±2.79 a 黑曲霉 32.18±0.13 cd 40.72±1.71 bc 30.45±1.40 c 26.87±2.13 a 灰孔多年卧孔菌 36.61±1.02 ab 33.96±0.67 d 59.77±0.90 a 29.66±1.91 a 安拉阿巴德篮状菌 + 黑曲霉 33.66±1.07 bc 39.91±2.99 bc 33.68±2.34 c 24.38±1.36 ab 安拉阿巴德篮状菌 + 灰孔多年卧孔菌 29.21±1.57 de 45.60±2.98 b 50.41±1.94 b 14.69±2.33 c 黑曲霉 + 灰孔多年卧孔菌 38.27±1.32 a 62.59±0.70 a 51.75±2.56 b 17.39±0.36 bc 说明:数值为平均值±标准差。同列不同小写字母表示不同处理组的降解率之间差异显著(P<0.05)。 Table 2. Dry matter and lignocellulose components degradation rates of rice straw
-
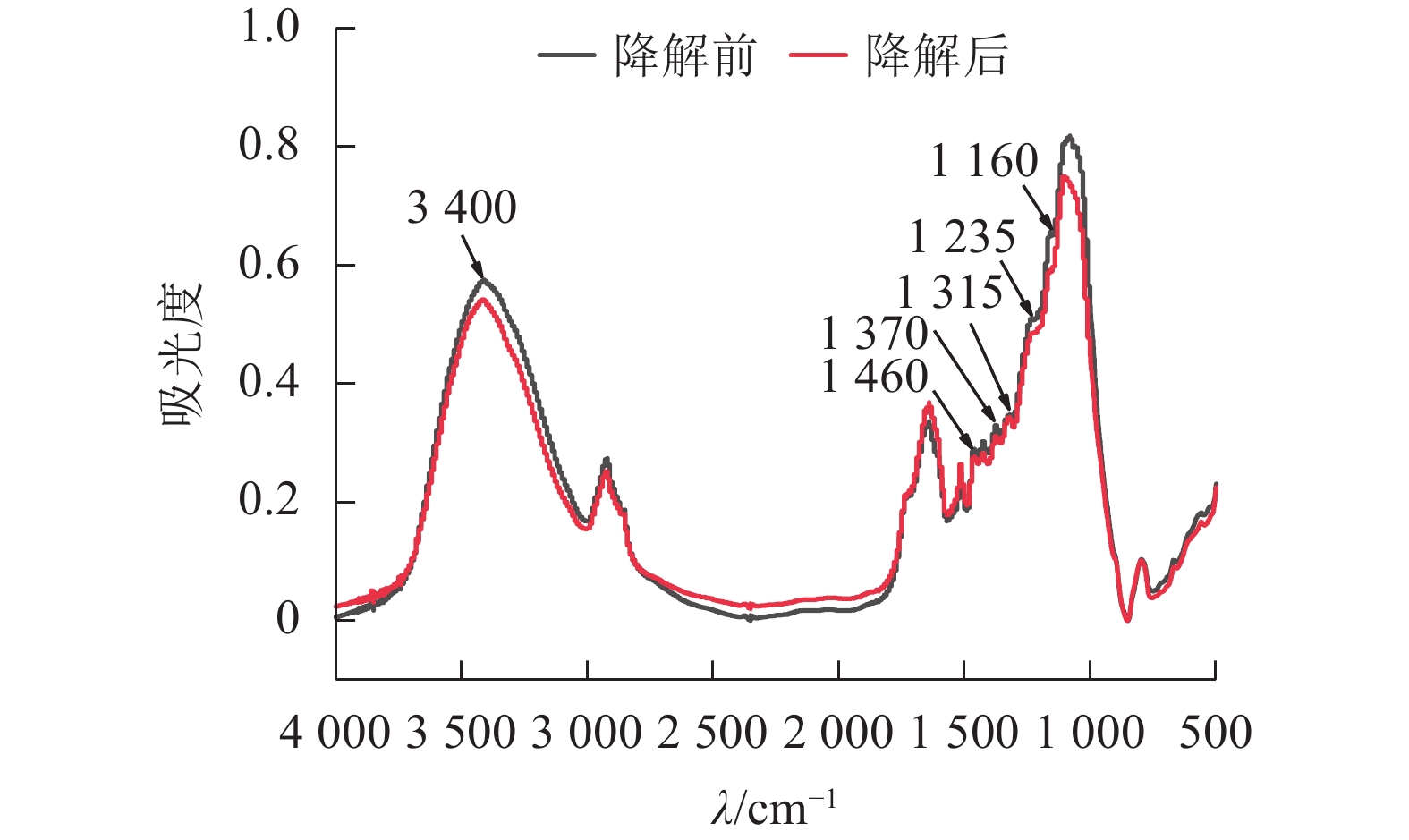
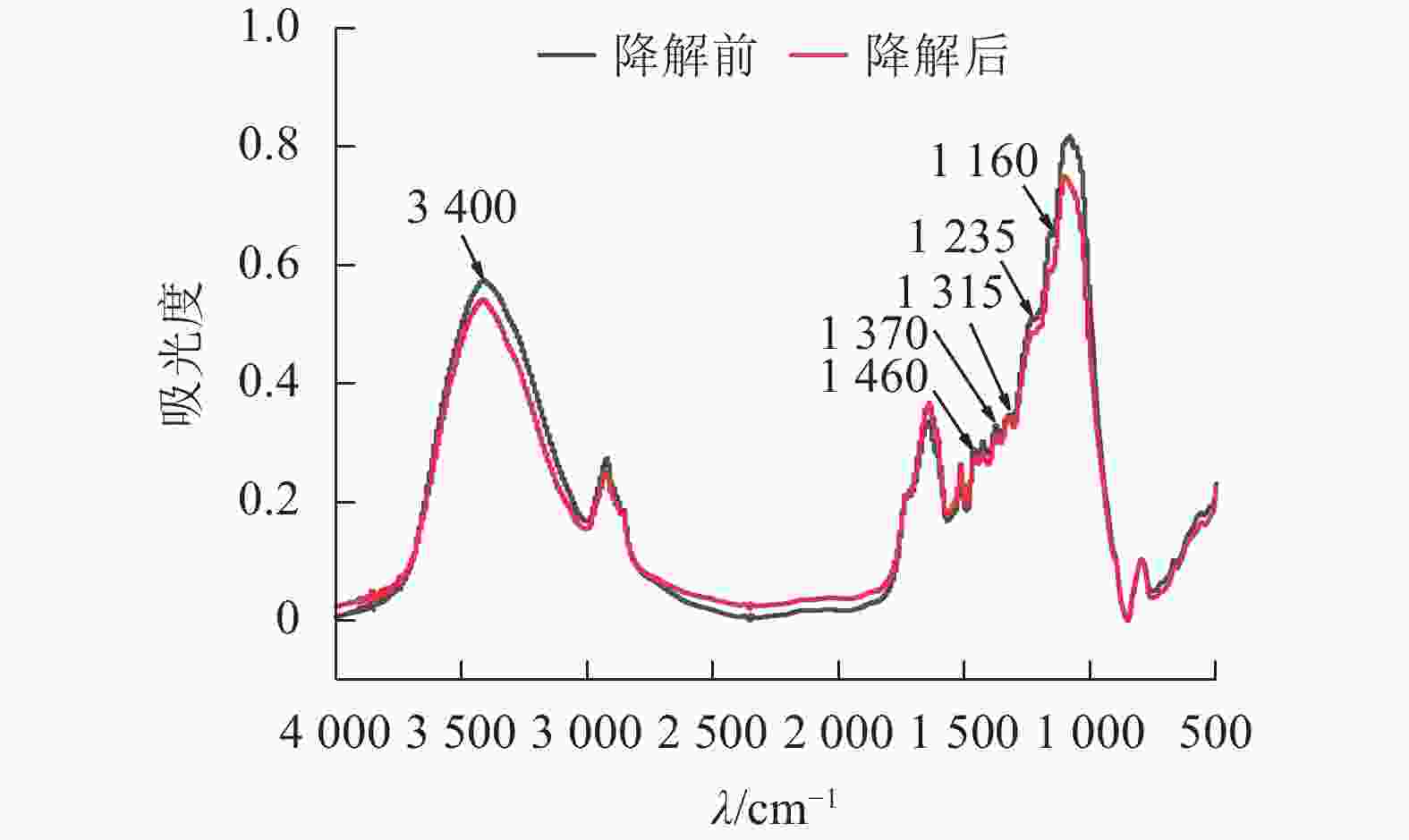
经黑曲霉与灰孔多年卧孔菌组合降解后,水稻秸秆部分红外吸收峰明显下降(图4),其中3 400 cm−1附近吸收峰与—OH伸缩振动相关[19],说明秸秆部分氢键被破坏,分子间作用力减弱;1 460 cm−1附近吸收峰与半纤维素中亚甲基(CH2)弯曲振动相关[19],表明真菌组合能有效破坏半纤维素结构;1 370和1 315 cm−1附近吸收峰分别与纤维素中CH2摇摆振动和C—H弯曲振动相关[20],证明真菌组合具有一定的纤维素分解能力;1 235 cm−1附近吸收峰与半纤维素-木质素复合物中O=C—C伸缩振动相关[19],说明部分复合结构被瓦解,半纤维素与木质素发生分离;1 160 cm−1附近吸收峰与碳链中C—O—C伸缩振动相关[19],表明在酶作用下,秸秆中部分碳链结构被破坏,分子聚合度下降。
-
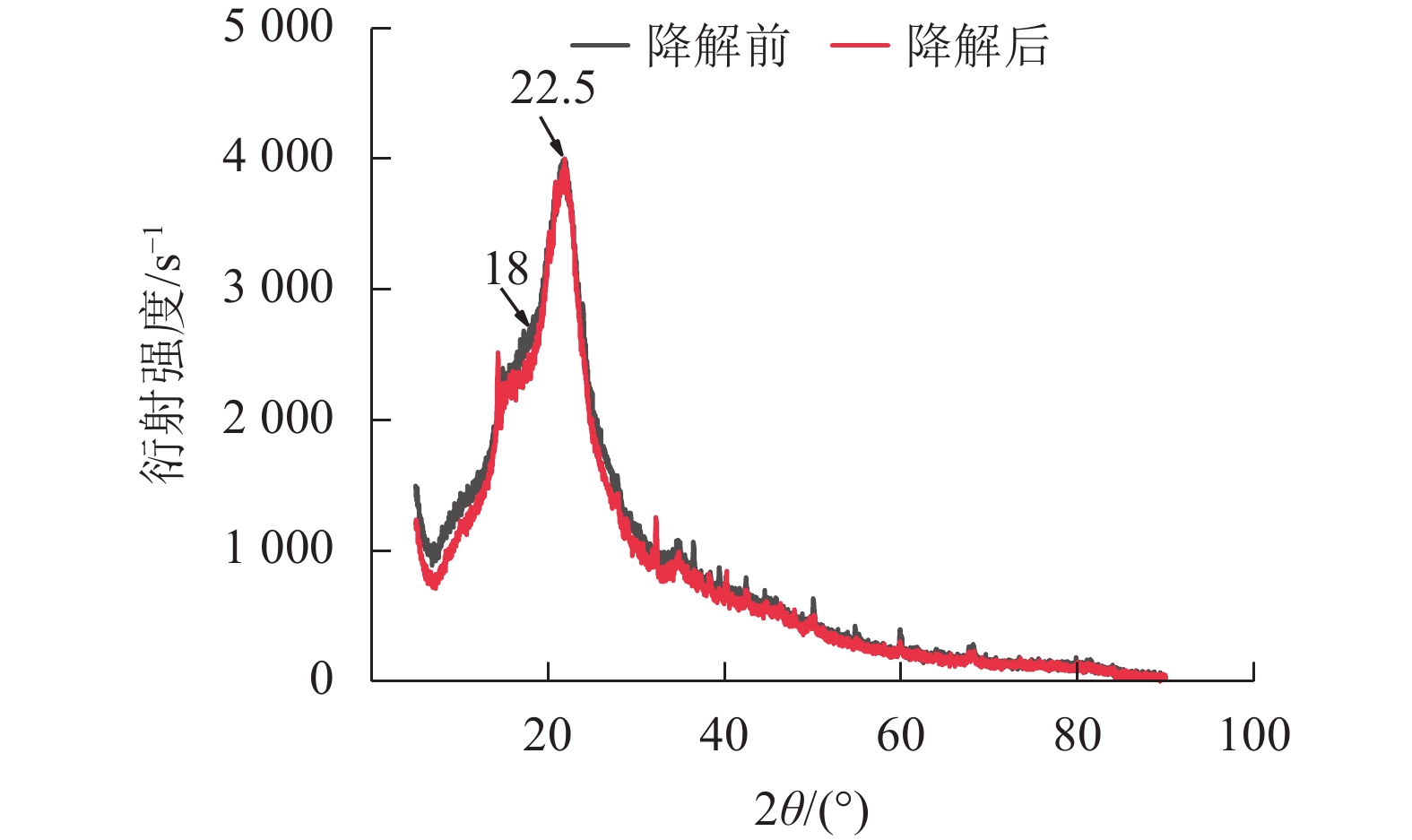
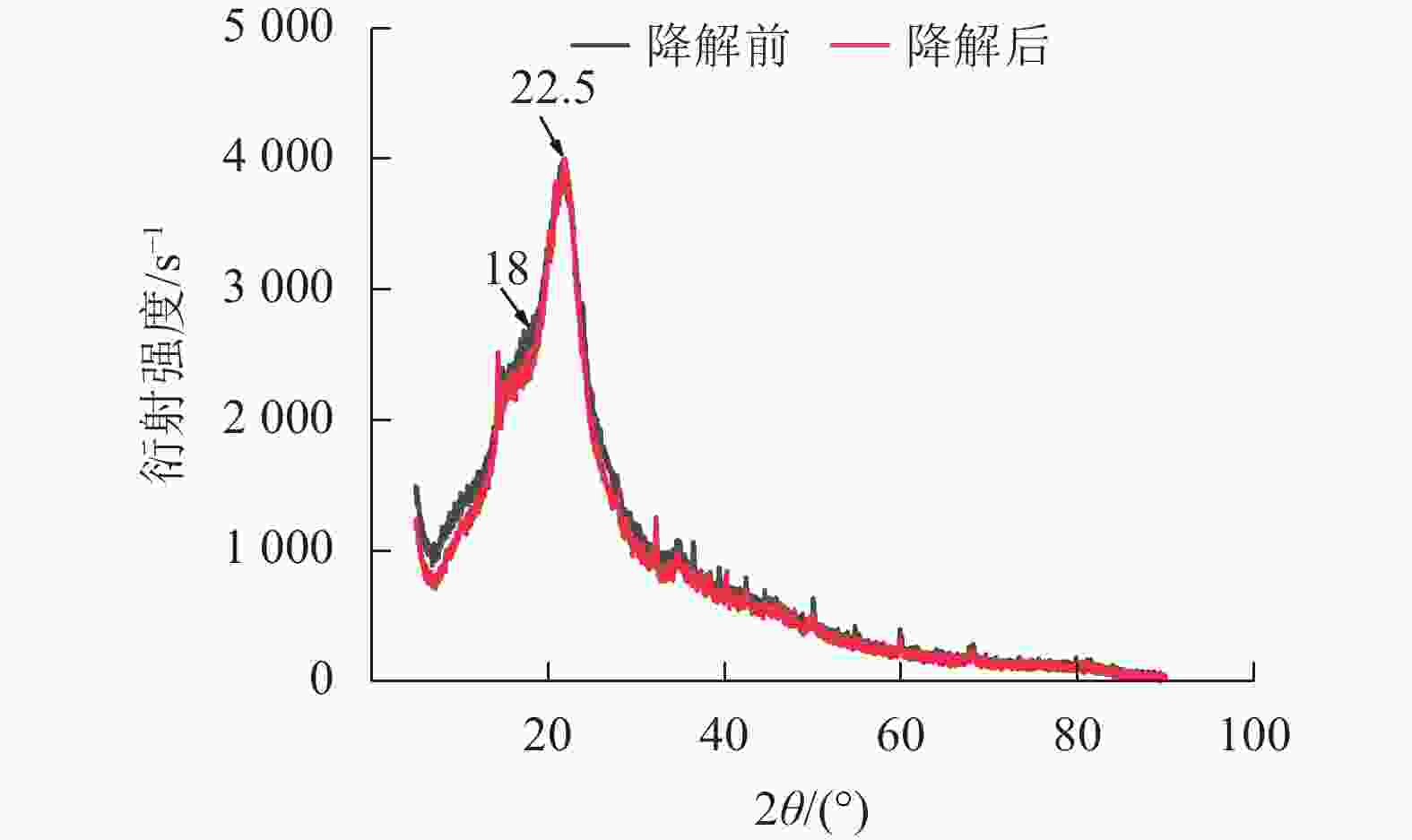
经黑曲霉与灰孔多年卧孔菌组合降解后,水稻秸秆纤维素非结晶区衍射峰(2θ = 18.0°)明显下降,纤维素结晶区衍射峰(2θ = 22.5°)无明显变化,水稻秸秆纤维素结晶度由22.44%上升至32.53%(图5)。说明相较于纤维素结晶区,真菌组合分泌的纤维素酶能够更容易分解纤维素非结晶区。但随着纤维素结晶度不断升高,纤维素整体晶体结构变为更加紧凑,推测对于进一步降解利用纤维素产生一定阻碍作用[20]。
-
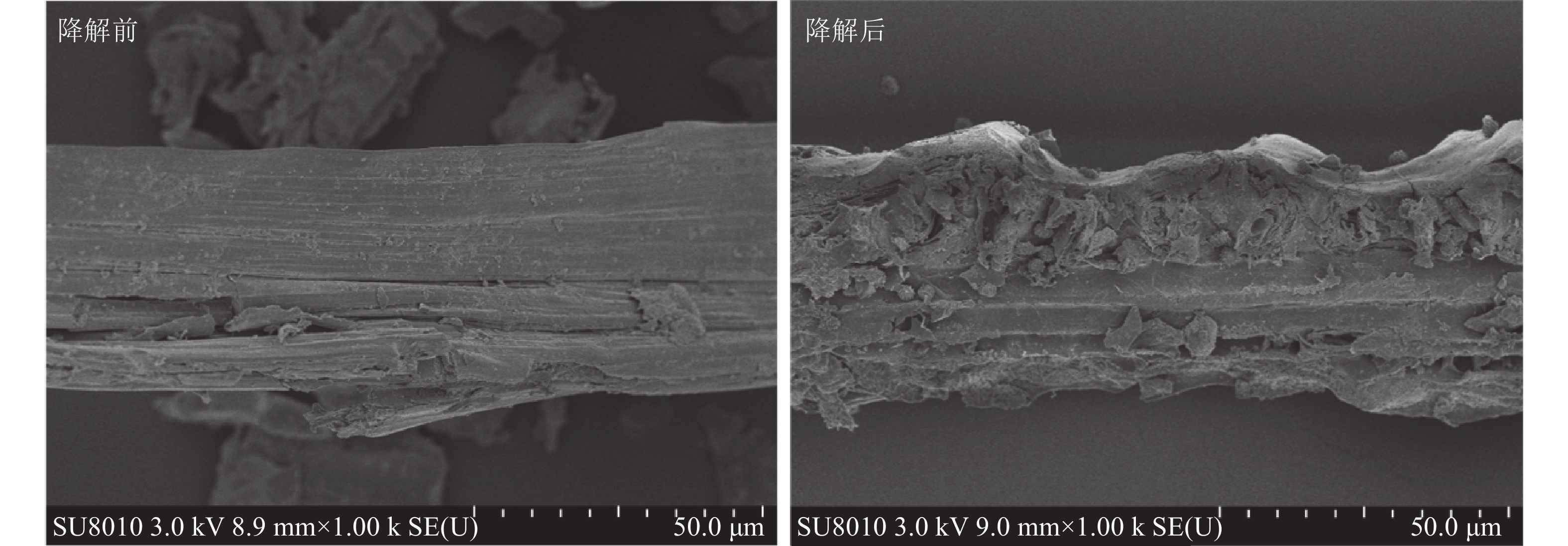
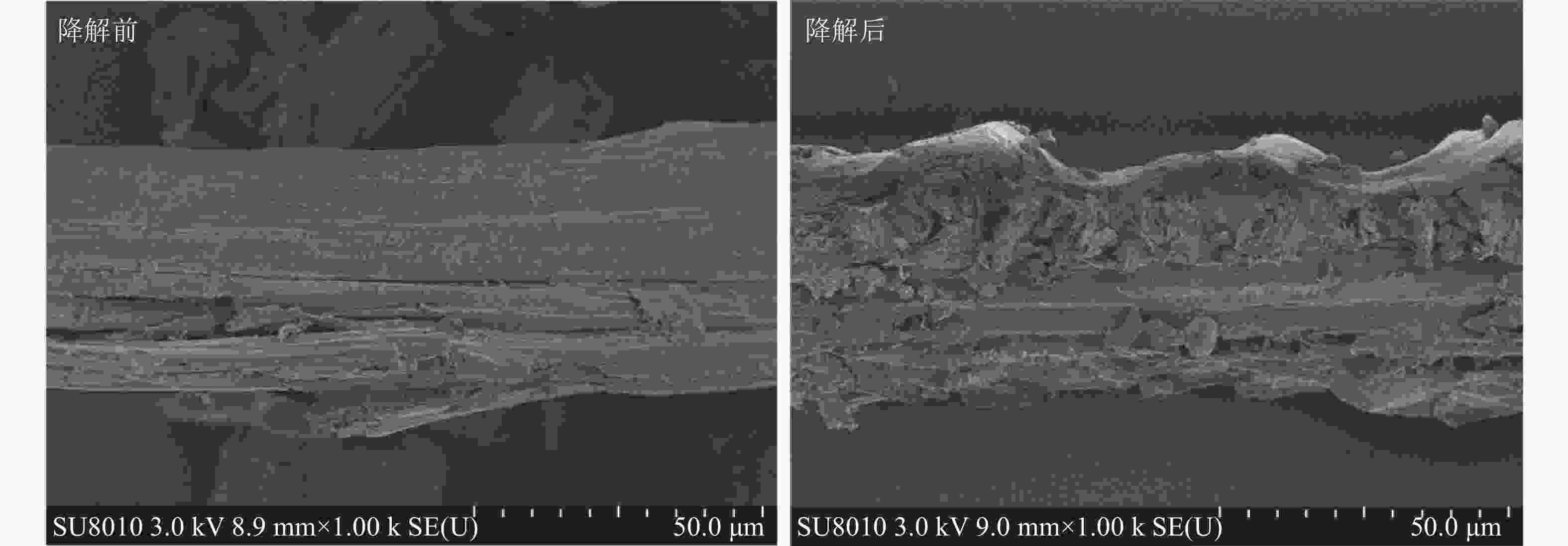
通过扫描电镜观察(图6),发现黑曲霉与灰孔多年卧孔菌组合降解后水稻秸秆表面由紧密光滑变为粗糙隆起,纤维结构崩解断裂,结构蓬松化,并产生大量孔隙和碎片,推测真菌菌丝可能通过孔隙深入秸秆内部,在内外双重降解下加速破坏秸秆结构。
-
随着分子生物学与环境生物学技术不断成熟,近年来利用真菌绿色降解水稻秸秆已取得了一定进展。MUSTAFA等[21]研究发现:糙皮侧耳和里氏木霉T. reesei对水稻秸秆纤维素、半纤维素和木质素降解率可分别达13.1%、23.7%、35.3%和20.1%、23.3%、23.6%。尹蕾等[22]从土壤中分离出的枝孢菌Cladosporium sp. BD-19可降解水稻秸秆中26.42%的纤维素、16.64%的半纤维素和11.43%的木质素。SHENG等[23]利用逆型金钱菌Gymnopus contrarius降解水稻秸秆后,去除了22.9%的纤维素、22.7%的半纤维素和41.9%的木质素。与上述研究相比,本研究从黑翅土白蚁肠道分离筛选的部分真菌具有更强的降解能力,其中刺孢篮状菌和黑曲霉具有更强的纤维素降解能力,降解率分别达38.49%和40.72%,灰孔多年卧孔菌具有更强的半纤维素降解能力,降解率达59.77%,推测这些共生真菌在白蚁消化木质食料过程中发挥一定协同降解作用。
在自然状态下,秸秆腐解往往是多种微生物共同参与的结果,将具有酶系互补作用的真菌组合培养不仅弥补部分菌种酶系不完整或个别酶活低的缺陷,同时还能减缓反馈抑制,从而提高降解效果[24]。江高飞等[25]研究发现:将草酸青霉等5种真菌复配后,复合菌系的秸秆降解能力和纤维素酶活性随着菌株种类增多而逐步升高。KOLASA等[26]将里氏木霉分别与黑曲霉等3种曲霉混合培养后,小麦Triticum aestivum秸秆降解率效果相较于单独降解均提升80%左右。本研究发现:当黑曲霉与灰孔多年卧孔菌“强强联合”后,水稻秸秆干物质降解率和纤维素降解率进一步提高,分别达38.27%和62.59%,同时半纤维素降解率仍保持较高水平,达51.75%,表现出极强的降解能力。进一步研究发现:两者组合能有效破坏水稻秸秆中半纤维素-木质素复合物内部化学键并减弱分子间作用力,推测在降解过程中纤维素、半纤维素和木质素三者间失去“黏性”,纤维素向外暴露,释放更多酶切位点供以降解[27−28]。本研究也证实:真菌组合更偏好分解纤维素非结晶区,推测是由于非结晶区吸水性更强,更容易与酶结合[29]。秸秆化学结构被破坏的同时,其物理结构也显著改变,降解后的秸秆表面崩解碎裂,结构蓬松化,产生大量碎片和孔隙,不仅有利于酶的渗透和吸附,真菌菌丝也更易探入内部“破坏”[30]。
-
本研究从黑翅土白蚁肠道分离筛选得到的安拉阿巴德篮状菌、刺孢篮状菌、黑曲霉和灰孔多年卧孔菌均具有水稻秸秆降解活性,其中黑曲霉和灰孔多年卧孔菌在组合降解水稻秸秆时表现出极强的降解能力,20 d内可降解水稻秸秆中38.27%的干物质、62.59%的纤维素和51.75%的半纤维素,并显著破坏秸秆理化结构,在秸秆生物降解产业化上具有潜在的开发价值。
Biodegradation of rice straw by symbiotic fungi of Odontotermes formosanus
doi: 10.11833/j.issn.2095-0756.20230140
- Received Date: 2023-02-06
- Accepted Date: 2023-05-19
- Rev Recd Date: 2023-04-23
- Publish Date: 2023-11-23
-
Key words:
- Odontotermes formosanus /
- symbiotic fungus /
- cellulase /
- utilization of straw resources
Abstract:
| Citation: | SHEN Yi, QI Mengwen, YANG Guiying, et al. Biodegradation of rice straw by symbiotic fungi of Odontotermes formosanus[J]. Journal of Zhejiang A&F University, 2023, 40(6): 1224-1231. DOI: 10.11833/j.issn.2095-0756.20230140 |









 DownLoad:
DownLoad: