-
玄武岩作为地球表面分布最广的岩石类型之一,其风化作用对于土壤形成和发育具有重要作用[1−2]。玄武岩中的矿物成分,如长石、辉石和橄榄石等[3],在风化过程中分解和转化,为土壤提供丰富的矿质营养元素[3−4],这些元素对于支持植物生长和生态系统功能至关重要[5−7]。许多研究表明:玄武岩风化在调节土壤地球化学以及地球气候变化等长期演化方面具有重要的作用[8−13]。如施用到土壤中的玄武岩粉末经风化作用后显著提高了土壤养分,促进了土壤有机碳的固定[14−15]。因此,研究玄武岩风化作用的影响因素对阐明土壤发生过程、矿质养分元素循环等具有重要作用。
土壤微生物通过代谢活动、化学作用、氧化还原作用以及与植物的共生关系等多种方式,加速土壤矿物的风化过程。其中,硅酸盐细菌不仅可以从矿物中提取经济价值较高的元素如钙(Ca)、镁(Mg)、锰(Mn),还可以作为生物肥料,从矿物中提取钾(K)、铁(Fe)、磷(P)等植物必需的营养元素[13−17]。与细菌相比,真菌在地质活动中的作用更加显著。有研究表明:真菌通过有机酸分泌、呼吸作用和络合物的生成等途径对玄武岩进行风化,参与风化的真菌主要包括地衣共生真菌、菌根真菌和腐生真菌[16−17]。
棘孢木霉Trichoderma asperellum和胶质芽孢杆菌Bacillus mucilaginosus是土壤中普遍存在的真菌与细菌,它们不仅能分解土壤中的难溶性矿物供自身的生长繁殖,还能促进土壤中矿质元素的地球化学循环[18−20]。棘孢木霉通过分泌有机酸和酶类,可以有效地促进矿物质溶解和元素释放,已有研究表明:它能加速硅酸盐矿物的风化,释放植物生长所需的硅元素(Si)[21−23]。而胶质芽孢杆菌则通过分泌多种有机酸和其他代谢产物,对钾长石等矿物的溶解效果显著,能够释放出K,提高土壤肥力。但是棘孢木霉和胶质芽孢杆菌的形态、大小和功能等具有各自的特点,因此它们在促进矿物风化的途径、机制等方面存在一定的差异[22−24]。
本研究选用了1株产酸量较大且能生成铁载体的棘孢木霉和1株硅酸盐细菌胶质芽孢杆菌,在恒温震荡培养条件下,探究它们对玄武岩风化过程中元素释放速率的影响,探讨其对玄武岩风化作用机制的异同,以期为不同菌种促进玄武岩风化提供理论依据。
-
选用的1株产酸量较大且能生成铁载体的棘孢木霉和1株硅酸盐细菌胶质芽孢杆菌,均由浙江农林大学省部共建亚热带森林培育国家重点实验室微生物研究室保存。棘孢木霉菌株生长迅速,在马铃薯葡萄糖琼脂培养基(potato dextrose agar, PDA)上生长时,营养体初体为白色的菌丝体,之后表面的颜色逐渐转变为青绿色,长势呈不定型棉絮状或致密丛束状。胶质芽孢杆菌菌株在Luria-Bertani (LB)培养基上形成隆起菌落,边缘整齐并凸起,中心有明显褶皱,革兰氏反应呈阴性。
-
PDA培养基用于活化培养棘孢木霉。LB培养基用于活化培养胶质芽孢杆菌。马铃薯葡萄糖培养基(PD)提供了统一且丰富的营养环境,确保细菌和真菌都能生长,将营养因素的影响降至最低。
-
玄武岩样品由嵊州皓达矿业有限公司提供。利用X-射线荧光光谱仪(X-ray Fluorescence Spectrometer,XRF)测定玄武岩的化学成分,其主要化学成分包括(质量分数):SiO2 47.27%,Al2O3 15.89%,Fe2O3 9.72%,MnO 0.57%,MgO 5.63%,CaO 1.28%,Na2O 5.03%,K2O 5.55%,TiO2 4.41%,P2O5 3.14%。
-
①棘孢木霉菌液的制备:挑起单菌落放入25 mL的PD培养基中,摇床上培养24 h。②胶质芽孢杆菌菌液的制备:挑起单菌落放入25 mL的PD培养基中,摇床上培养24 h,5 000 r·min−1离心6 min,去除上层清液,底层沉淀物用无菌水打散,即为细菌菌液。
-
分别称取5.0 g的玄武岩岩粉放置于250 mL的锥形瓶,每瓶加入120 mL的PD培养基,在115 ℃高压灭菌锅灭菌30 min。然后分别加入3 mL的棘孢木霉菌液、胶质芽孢杆菌菌液和无菌水(对照),处理的编号分别为MM、YB、ck,并用厚度为0.2 μm的半透膜封口,以保证瓶内处于有氧环境,每个处理3次重复。将所有的锥形瓶放置于28 ℃、150 r·min−1的恒温振荡培养箱内。根据细胞的生长曲线,在第1、3、7、15、20、30天共6次取样,每次从锥形瓶中取2 mL的浸提液用于测定。
-
浸提液pH值和离子浓度[25]:浸提液pH用台式pH计在室温下测定,精度±0.01。Si、Ca,Mg,Fe和铝(Al)的浓度用电感耦合等离子体发射光谱法(ICP-OES)测定,在测定前将浸提液过0.22 μm的醋纤滤头,按照时间序列,以不同的倍数用体积分数为3%的0.05 mol·L−1HNO3稀释至3 mL, 3次重复,取平均值,其标准偏差(SD)<5%。为减小实验误差,用公式(1)对所测数据矫正。
式(1)中,$ {C}_{j,i}^{\mathrm{*}} $ 是离子i在第j次取样后的矫正浓度(mmol·L−1),$ {v}_{0} $是反应体系溶液的初始体积(120 mL),$ {C}_{j,i} $ 是测定浓度,$ {v}_{s} $ 是每次取样的体积(2 mL),$ \displaystyle\sum _{h=1}^{j-1}{C}_{j,i}{v}_{s} $ 是取样过程中提取离子i的质量。
用线性溶解速率来表征元素释放快慢,具体参照公式(2)计算。
式(2)中,$ {R}_{i}^{l} $是离子i的线性释放速率(mol·m−2·s−1),$ \mathrm{d}{C}_{i}^{\mathrm{*}}/\mathrm{d}t $是离子i矫正后浓度对于时间t的微分,v0是反应体系溶液的初始体积(120 mL),A为玄武岩粉末的比表面积(
0.2437 ±0.0027 ) m2·g−1,m为玄武岩样品的质量(5.0 g)。采用冷场发射扫描电子显微镜(CFESEM)对反应后的玄武岩颗粒表面形态进行分析,并根据能谱仪(EDS)形成的能谱图的能量值确定元素种类。
-
采用Excel 2019和Origin 2020进行数据整理和作图分析。
-
如图1所示:与ck相比,MM和YB处理对浸提液的pH产生了影响,其中MM组浸提液的pH呈“降低—上升”的趋势,YB组浸提液的pH呈“降低—上升—降低”的趋势。在第7天,MM组的pH达到最低点,低于ck (1.46),在第15~30天呈现明显的升高趋势,高于YB组0.96。YB组在第7天时浸提液的pH达到最低点,低于ck (0.88)。随着时间的推移,YB组的pH呈现微小的变化趋势,并且整个过程pH均低于ck。
-
如图2所示:MM组浸提液中各元素的最高浓度高于ck,Si、Ca、Al、Fe、Mg的最高浓度分别是ck的10.2、2.6、8.2、92.9和9.9倍;YB组中Si、Ca、Al、Fe、Mg的最高浓度分别是ck的2.7、1.2、1.7、19.7和3.2倍。MM组Si、Ca、Al、Fe和Mg的最大释放量分别是YB的3.3、1.9、6.0、5.0和3.2倍。
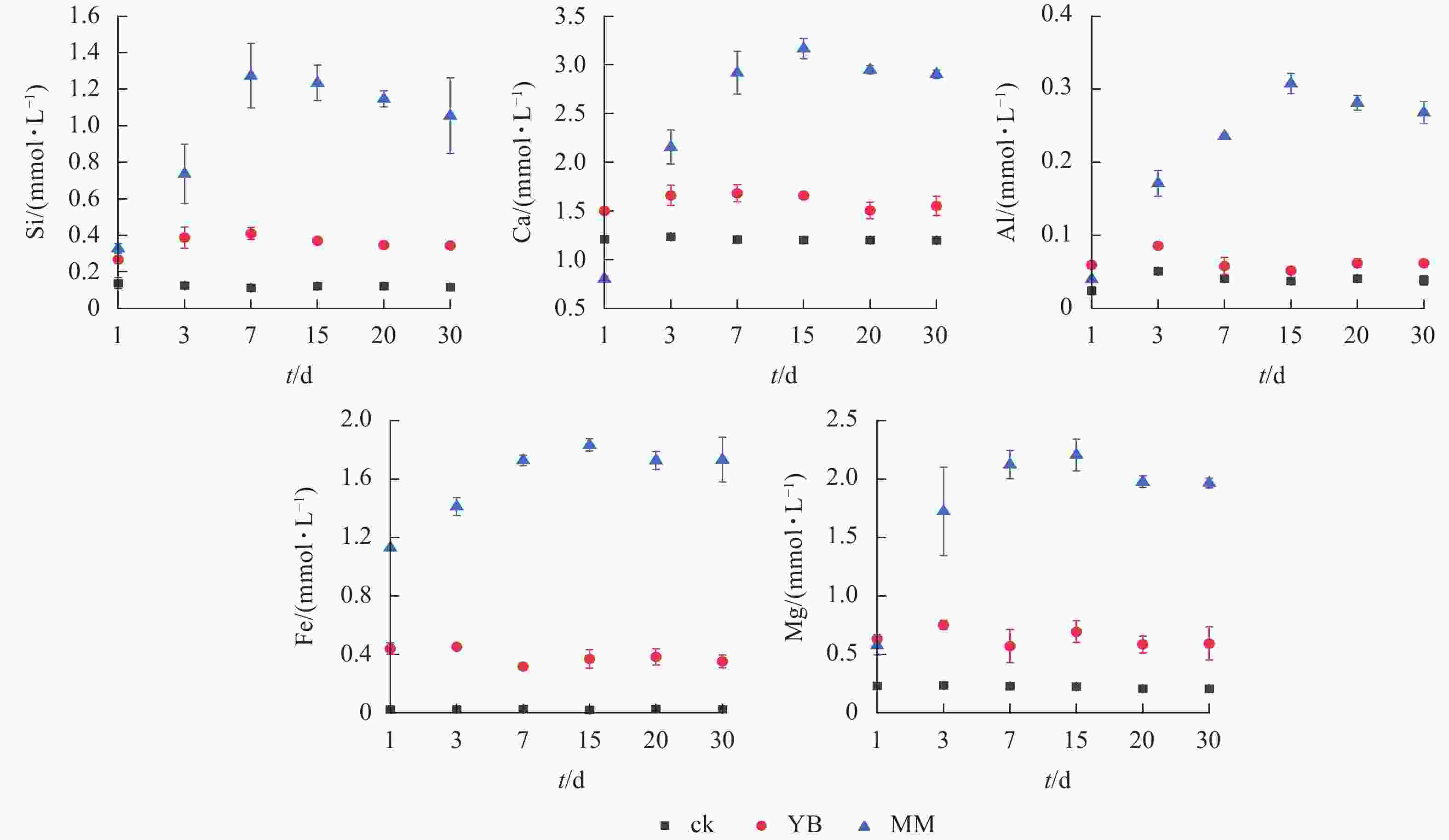
Figure 2. Dynamic variations in the concentrations of Si, Ca, Al, Fe, and Mg in the leaching solution
在MM组中,Si、Ca、Al、Fe和Mg的浓度表现出波动,总体趋势升高,其中,Si的浓度在第7天达到最大值,而Ca、Al、Fe和Mg则在第15天达到最高浓度,这表明在第7~15天风化效果最佳。在YB组中,元素浓度波动幅度小于MM组。综上所述,胶质芽孢杆菌和棘孢木霉都能够风化玄武岩,并释放其中的矿质元素,且棘孢木霉的风化效果更好。
-
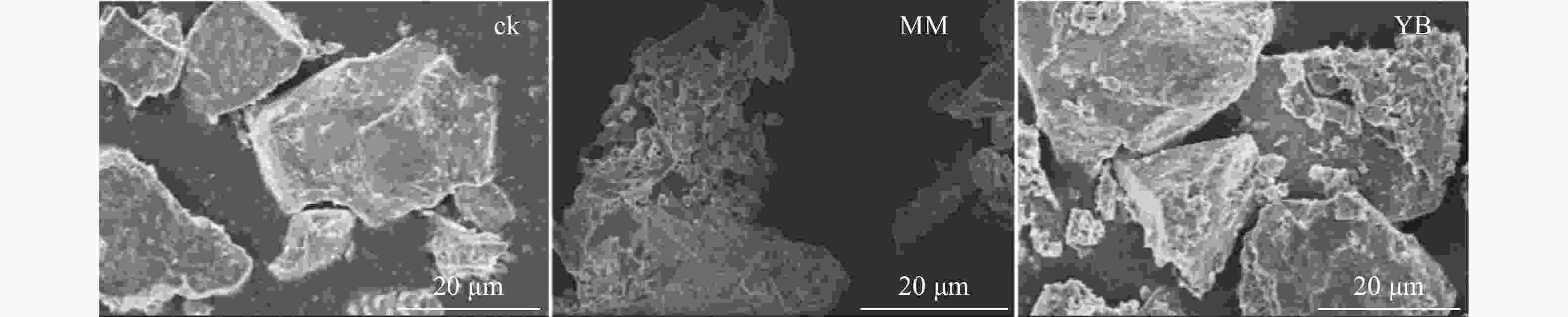
电子显微镜扫描(图3)结果表明:棘孢木霉和胶质芽孢杆菌所在体系中,玄武岩颗粒表面均呈明显的不规则碎片,相反,在ck中,玄武岩表面保持光滑,没有明显的风化现象。
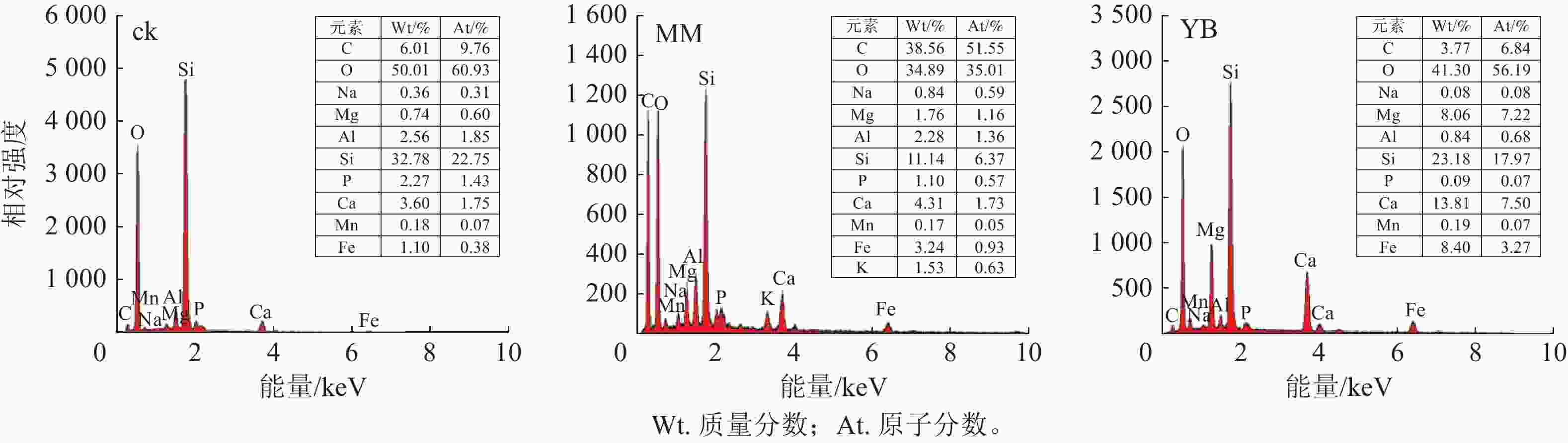
能谱分析结果(图4)表明:经过30 d反应的玄武岩残渣表面主要元素为碳(C)、O、Si、Al等,其中C可能来源于培养基和微生物残体。同时,玄武岩表面还附着了其他元素,包括Mn、钠(Na)、Mg、Al、P、K、Ca和Fe等,其中MM组的元素浓度高于YB组。说明微生物参与了促进玄武岩风化并释放矿质养分的过程,且棘孢木霉在这个过程中发挥了明显的作用。
-
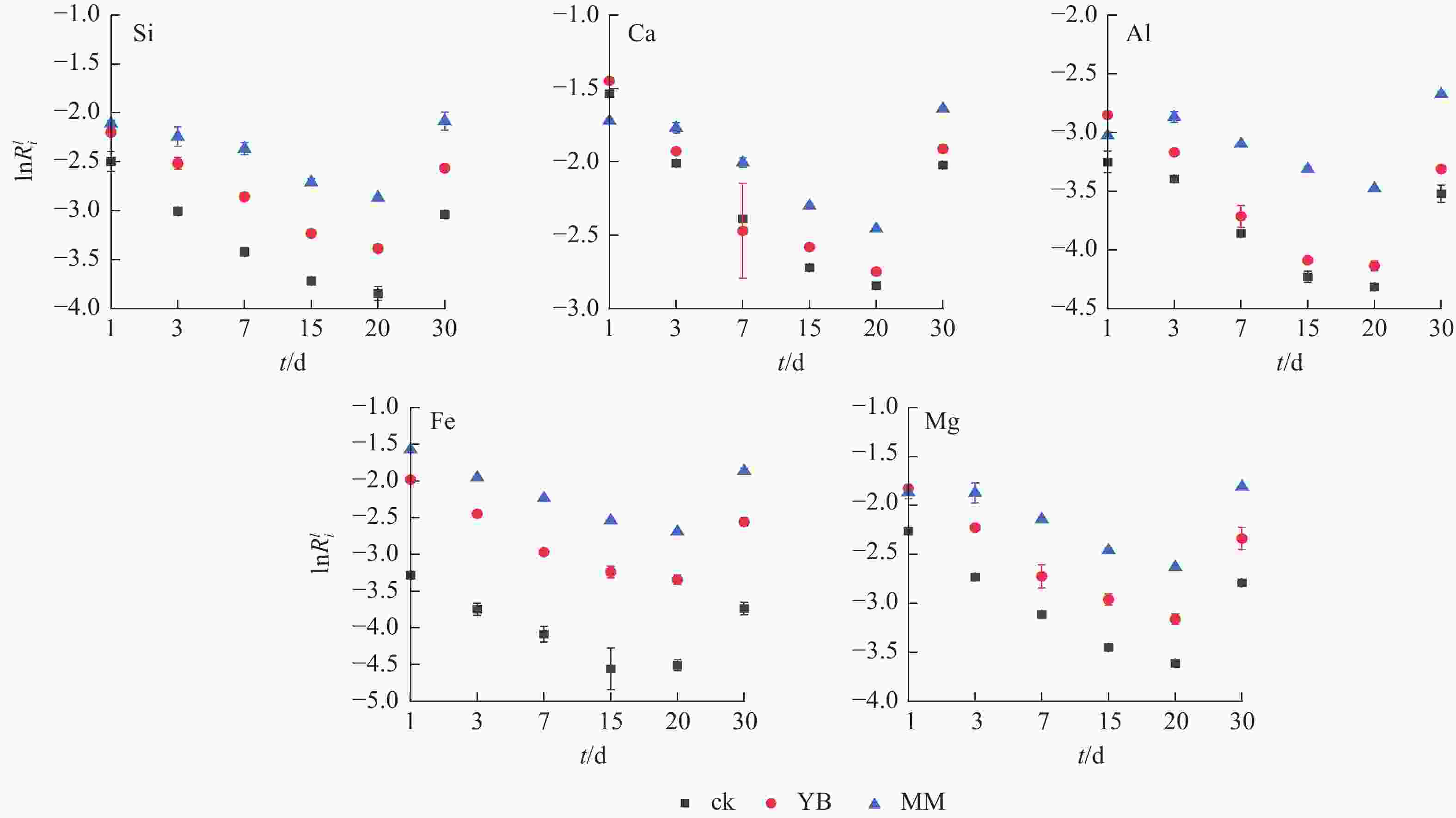
如图5所示:随着时间的推移,这3个体系的元素线性溶解速率表现为先下降后上升,且在第20天达到最低值。MM组中的Ca、Mg和Al的线性溶解速率在第3天有个突然升高点,Fe的变化趋势相对其他元素较稳定。另一方面,YB组中Ca和Al溶解速率在第1天高于MM组,但随着时间的延长,逐渐降低,低于MM组。在MM组中,Si、Ca、Al、Fe和Mg的释放速率高于胶质芽孢杆菌0.49、0.28、0.78、0.70和0.50 mol·m−2·s−1,除Ca外,其他元素的线性溶解速率从高到低依次为MM、YB、ck,表明微生物加速了矿物元素的释放,刺孢木霉的参与对元素释放速率的效果更好。
-
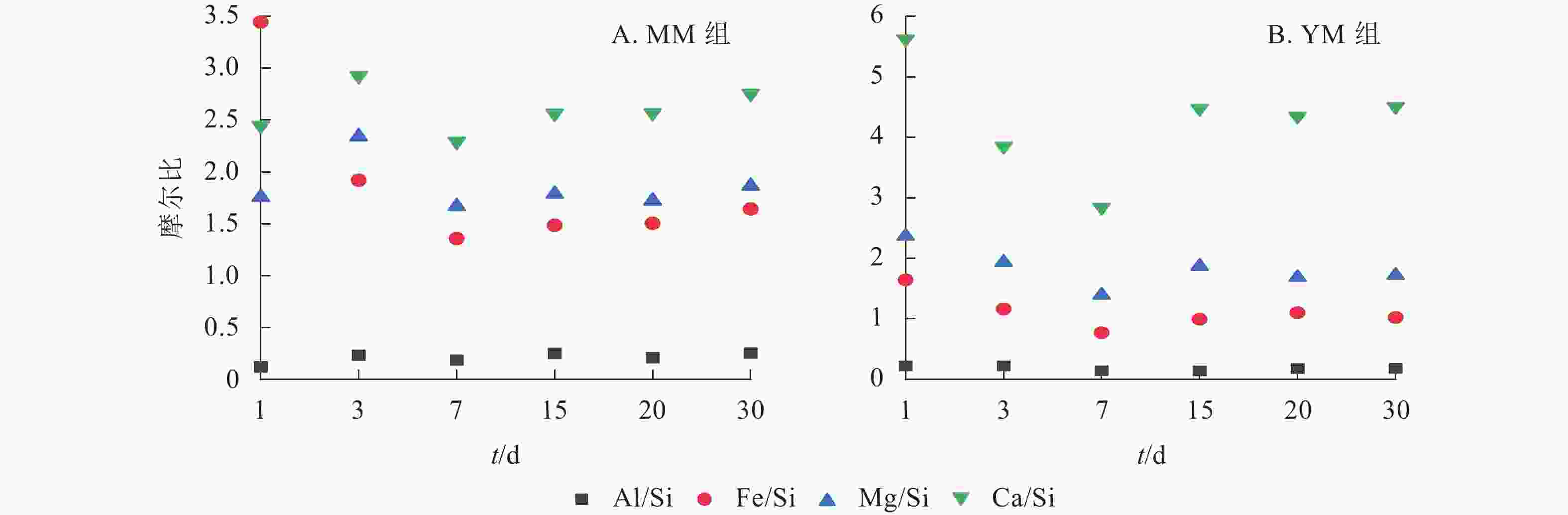
为了明确元素的释放能力顺序,对MM和YM组中的Al/Si、Fe/Si、Mg/Si、Ca/Si摩尔比进行对比分析,结果如图6所示。在不同体系中,各元素与Si的摩尔比值从大到小依次为Ca/Si、Mg/Si、Fe/Si、Al/Si。然而,MM组中Fe/Si的摩尔比在1~3 d内呈下降趋势,这主要是因为Si的释放量较低。玄武岩样品的Al/Si、Fe/Si、Mg/Si、Ca/Si摩尔比分别为0.336、0.206、0.119、0.027。通过比较浸提液中元素的硅浓度最大值所对应的摩尔比值与玄武岩样品中的相应比的倍数,发现元素释放能力的排序:在MM组中,从大到小排序为Ca (84.9)、Mg (14.0)、Fe (6.6)、Si (1.0)、Al (0.6);在YM组中,从大到小排序为Ca (105.2)、Mg (11.7)、Fe (3.7)、Si (1.0)、Al (0.4)。
-
微生物能够栖居于岩石表面已经得到大量研究证实[26]。相关研究发现:细菌和真菌展现了出色的适应能力和显著的地质活动,通常形成地衣共生,并广泛分布于陆地岩石圈表面[27−28]。为了明确不同菌株对玄武岩风化作用强度的影响,本研究对真菌和细菌浸提液中矿物元素释放量和释放速率进行比较,发现棘孢木霉组的风化速率高于胶质芽孢杆菌组。本研究棘孢木霉组中,pH为5.16~7.44,而在胶质芽孢杆菌组中pH为5.88~6.26,真菌的pH波动幅度大且在长时间内低于细菌组,表明棘孢木霉能够分泌更多的酸,从而促进元素的溶出速率,这与王浩贤等[25]的研究结果一致。棘孢木霉组pH在后期显著上升可能原因有:①随着玄武岩的风化,释放出大量的碱性盐基离子(Ca2+、Mg2+、K+、Na+),从而中和酸性;②后期真菌细胞自溶,氨基氮含量增加,造成pH上升;③随着时间的推移,有机酸可能被分解或进一步代谢,减少了溶液中的酸性成分,从而导致pH上升[27]。真菌的产酸量被证明是细菌的200多倍[29],突显了真菌在促进元素溶出方面的高效性。这对于理解岩石风化机制以及土壤养分释放过程具有重要的启示。
在棘孢木霉组和胶质芽孢杆菌组中,元素释放量从高到低依次是Ca、Mg、Fe、Si、Al。其中,Ca的释放量最高,为3.17 mmol·L−1,说明Ca相对于其他元素更容易溶出,周跃飞等[30]的研究中也证明了这点。其原因是真菌和细菌在代谢活动中引发了生物地球化学反应,加速了含有Ca的矿物分解并释放到溶液中。本研究还发现:棘孢木霉组中变化最大的是Fe,其原因可能是在真菌的参与下,Fe发生了较为明显的络合作用。LI等[31]研究表明:真菌能通过Fe载体显著提高矿物中Fe释放。SANTHIYA等[32]研究表明:微生物在生长繁殖过程中产生多聚糖类物质,不仅促进质子交换,还能通过配体络合作用促进矿物的溶解,在这些矿物中,Fe、Mg等元素也容易形成络合物[33−34]。
-
不同菌株对玄武岩风化过程中元素释放能力的影响因素较多,在试验过程中可能会存在一些影响因素[35],如释放出的矿质元素可能被微生物吸收成为细胞内酶的组成部分,或被真菌菌丝所吸附;释放出的Mg可能以水合离子的形式存在于溶液中;根据RAMOS等[36]的研究,当pH>6.5时,浸提液中的部分Al可能以Al(OH)3的形式存在,然而,在本研究真菌(棘孢木霉)组的pH在6.06~7.44内变化,可能导致Al低于实际释放量,Fe也可能出现类似情况;从生物量的角度看,真菌的生物量远远大于细菌,在生长发育过程中可能吸收了更多的矿质元素,因此元素释放能力真菌实际远远高于细菌。这些情形说明浸提液中的元素实际上只是元素释放量的一部分。但是Ca/Si的摩尔比值仍远远高于Mg/Si,因此真菌和细菌的作用下,元素的实际溶出能力从大到小仍然是Ca、Mg、Fe、Si、Al。
-
微生物栖居于岩石表面进行活动会代谢产生一部分质子(H+),通过H+与岩石矿物表面的金属阳离子发生交换,会促使矿物溶解[37]。本研究发现:棘孢木霉和胶质芽孢杆菌体系中浸提液的pH变化呈不同趋势,其原因是棘孢木霉的介入初期能够分泌较多酸,从而降低其pH,而随时间推移,真菌促进玄武岩风化并释放盐基离子(Ca和Mg)导致pH提高;胶质芽孢杆菌在风化玄武岩的过程中释放了酸性物质,这些酸性物质逐渐电离出H+,进而导致浸提液pH降低[28],这一现象与李永等[29]在使用细菌风化岩石时观察到pH降低的情况相符。此外,无论是棘孢木霉还是胶质芽孢杆菌,浸提液pH在刚开始时均低于对照,这说明真菌和细菌均有可能释放出了酸,营造酸性环境。本研究元素浓度动态变化结果表明:棘孢木霉组的Si、Ca、Al、Fe、Mg等5种元素的浓度均高于胶质芽孢杆菌组和对照,且线性释放区间更长。与pH变化结合,发现在这个时间段棘孢木霉组的pH呈直线下降,第7天pH最低,同时Mg和Fe元素的浓度变化呈现相似趋势,但Fe较为平稳的上升,这表明微生物能通过代谢一些酸性物质营造酸性环境而促进元素的溶出,这与STONE[38]的研究结果一致。
胶质芽孢杆菌组矿物元素的浓度总体低于棘孢木霉组,但高于对照。其中,Si、Ca、Al、Fe和Mg等5种元素的浓度在一定范围内呈相对平稳的变化趋势。结合pH图对比分析,胶质芽孢杆菌组的溶液pH低于对照,说明矿物元素的释放受酸性环境的控制,其原因是真菌和细菌在与岩石相互作用的过程中会分泌不同种类的有机酸,导致周围环境酸化,并提供质子以加速矿物的溶解。这些有机酸还通过络合作用加速矿物表面元素的释放[39]。相关研究表明:Si、Al、Fe、Mg等元素易与有机酸形成络合物,促进矿物中元素的溶解[40−41]。棘孢木霉相较于胶质芽孢杆菌能分泌更多的有机酸,能更有效地促进玄武岩矿物元素的释放。
-
棘孢木霉和胶质芽孢杆菌能明显加速玄武岩的风化进程,且棘孢木霉处理下的玄武岩风化速率明显高于胶质芽孢杆菌处理下的风化速率,这表明真菌在矿物风化过程中的能力优于细菌。棘孢木霉和胶质芽孢杆菌均提高了Si、Ca、Al、Fe和Mg元素的释放,且以棘孢木霉的提高效果最好。棘孢木霉和胶质芽孢杆菌作用下,玄武岩中元素的释放能力从大到小均依次为Ca、Mg、Fe、Si、Al。这表明微生物种类可能对元素的溶出能力影响不大,真菌和细菌对促进玄武岩风化的机制可能相似。
Effects of different microbial strains on element release during weathering of basalt
doi: 10.11833/j.issn.2095-0756.20240381
- Received Date: 2024-06-04
- Accepted Date: 2024-08-21
- Rev Recd Date: 2024-07-12
- Available Online: 2025-05-30
- Publish Date: 2025-05-30
-
Key words:
- basalt /
- Trichoderma asperellum /
- Bacillus mucilaginosus /
- weathering /
- release rate /
- mechanism
Abstract:
| Citation: | YANG Pan, LUO Yubo, YANG Jiao, et al. Effects of different microbial strains on element release during weathering of basalt[J]. Journal of Zhejiang A&F University, 2025, 42(3): 468−476 doi: 10.11833/j.issn.2095-0756.20240381 |











 DownLoad:
DownLoad:




