-
光质在植物生长发育过程中具有重要作用,不仅影响种子萌发、光合作用、形态建成与花芽分化[1−3],还可通过调控有机物积累影响果蔬品质[3−5]。在单色光中,红光可提高莴苣Lactuca saliva[6]、葡萄Vitis vinifera[7]和红叶桃Prunuspersicaf atropurpurea[8]叶片中的叶绿素含量,蓝光可促进类胡萝卜素的积累[9],而绿光则不利于光合色素的合成与积累[10]。与蓝光相比,红光不仅可提高茄子Solanum melongena[11]与番茄S. lycopersicum[12]的光合速率,还有利于茄皮积累类黄酮[13]。与白光相比,红光不仅可促进豌豆Pisum sativum芽苗菜生长,还可提高其叶绿素、粗纤维和可溶性糖含量[14]。在白光、红光、蓝光、绿光、黄光和复合光中,红光最有利于提高樱桃番茄Lycopersicon esculentum var. cerasiforme的产量以及果实中番茄红素与可溶性糖含量,而绿光最不利于其产量与品质的提升[15]。与蓝光相比,红光可显著提高辣椒Capsicum annuum果实中的可溶性糖含量[16]。与白光相比,红光可通过诱导光敏色素基因表达,从而促进光信号传递以诱导马铃薯S. tuberosum中糖的苷生物碱合成与积累[17]。此外,红光还可通过诱导转录因子表达,从而促进类胡萝卜素合成与果实转色[18]。由此可见,红光对提升果蔬光合性能与果实品质均具有促进效应。
在红、蓝、黄、绿、紫和透明膜中,红膜不仅可促进草莓Fragaria × ananassa植株生长,提高光合色素含量与光合性能[3],还可通过诱导醇酰基转移酶与橙花叔醇合酶基因表达以促进酯类和反式-橙花叔醇等香气成分形成[19]。MIAO等[20]研究发现:红膜可通过提高草莓花青素合成相关酶活性以促进花青素积累,而绿膜和蓝膜则具有抑制效应。对大白菜Brassica rapa subsp. pekinensis而言,红膜和紫膜可通过提高其光合性能以促进植株生长,进而提升产量[21]。此外,红色遮阳网处理也能促进番茄植株与叶片生长,并提高葡萄糖含量、果糖含量、糖酸比、单果质量等果实品质[22]。
黄瓜Cucumis sativus、南瓜Cucurbita moschata和辣椒是重要的瓜果类蔬菜,可在设施大棚中常年种植。鉴于红膜(光)对果蔬生产具有明显的促进效应,本研究采用2种红膜分别搭建大棚,探究红膜对黄瓜、南瓜、辣椒光合性能与果实品质的影响,为利用适宜红膜促进瓜果类蔬菜生产提供参考。
-
2024年3月,在浙江省杭州市临安区后营村后营蔬菜生产基地(30o11′27″N,119o12′10″E),分别采用2种红膜(RF1和RF2)与普通棚膜(ck,厚度均为80 μm)搭建大棚(长×宽×高为32.0 m×8.0 m×3.3 m),各棚膜光谱特征如图1所示。在每个大棚内分别种植黄瓜‘浙秀3号’ ‘Zhexiu No. 3’、南瓜‘新栗1号’‘Xinli No. 1’和辣椒‘杭椒早秀’‘Hangjiao Zaoxiu’,定植时叶片数量分别为3、2和9片,种植数量分别为200、30和280株。以在普通棚膜大棚种植作为对照。在种植期间进行常规栽培管理,待种植约60 d进入盛果期后,各处理随机选取6~10株进行光合性能与果实品质测定,每株视为1次重复。
-
蔬菜叶片经暗适应30 min后,采用YZQ-500叶绿素荧光仪测量叶绿素荧光诱导动力学曲线,计算光系统Ⅱ (PS Ⅱ)最大光化学量子产量(φPo)、捕获的激子所导致的电子传递效率(Ψo)、电子传递的量子产额(φEo)与非光化学猝灭的最大量子产量(φDo)[23−24]。
-
参考PENG等[19]的方法,称取2 g蔬菜果实,用6 mL体积分数为80%的乙醇研磨成匀浆。经8 000 r·min−1离心后,上清液用于测量果糖、葡萄糖、蔗糖、还原糖和总糖质量分数。其中,果糖和蔗糖均通过加入蒽酮试剂进行测定,并分别用果糖和蔗糖标准品制作标准曲线以计算相应糖质量分数。还原糖通过加入3,5-二硝基水杨酸进行测定,并利用葡萄糖制作标准曲线以计算还原糖质量分数。果实中葡萄糖质量分数=还原性糖质量分数−果糖质量分数,总糖质量分数=蔗糖质量分数+还原糖质量分数。
-
称取1 g果实并用2 mL无水乙醇研磨成匀浆,经5 000 r·min−1离心15 min后,取1 mL 上清液并加入0.5 mL 0.5 mol·L−1福林酚、1.5 mL 0.765 mol·L−1Na2CO3溶液。在暗处反应2 h后,测量溶液在765 nm处的吸光度。以没食子酸为标准品制作标准曲线,计算果实中总酚质量分数[25]。
-
称取4 g果实并研磨成匀浆,经8 000 r·min−1离心后,采用阿贝折射仪测量可溶性固形物质量分数[25]。
-
称取0.2 g果实,加入pH 7.8磷酸钾缓冲液以研磨成匀浆。经8 000 r·min−1离心后,取1 mL上清液并与4 mL考马斯亮蓝G-250溶液混匀,于595 nm处测量其吸光度。采用牛血清蛋白制作标准曲线,计算果实中可溶性蛋白质量分数[26]。
-
采用Excel整理数据,利用Origin 2024进行单因素方差分析并作图。
-
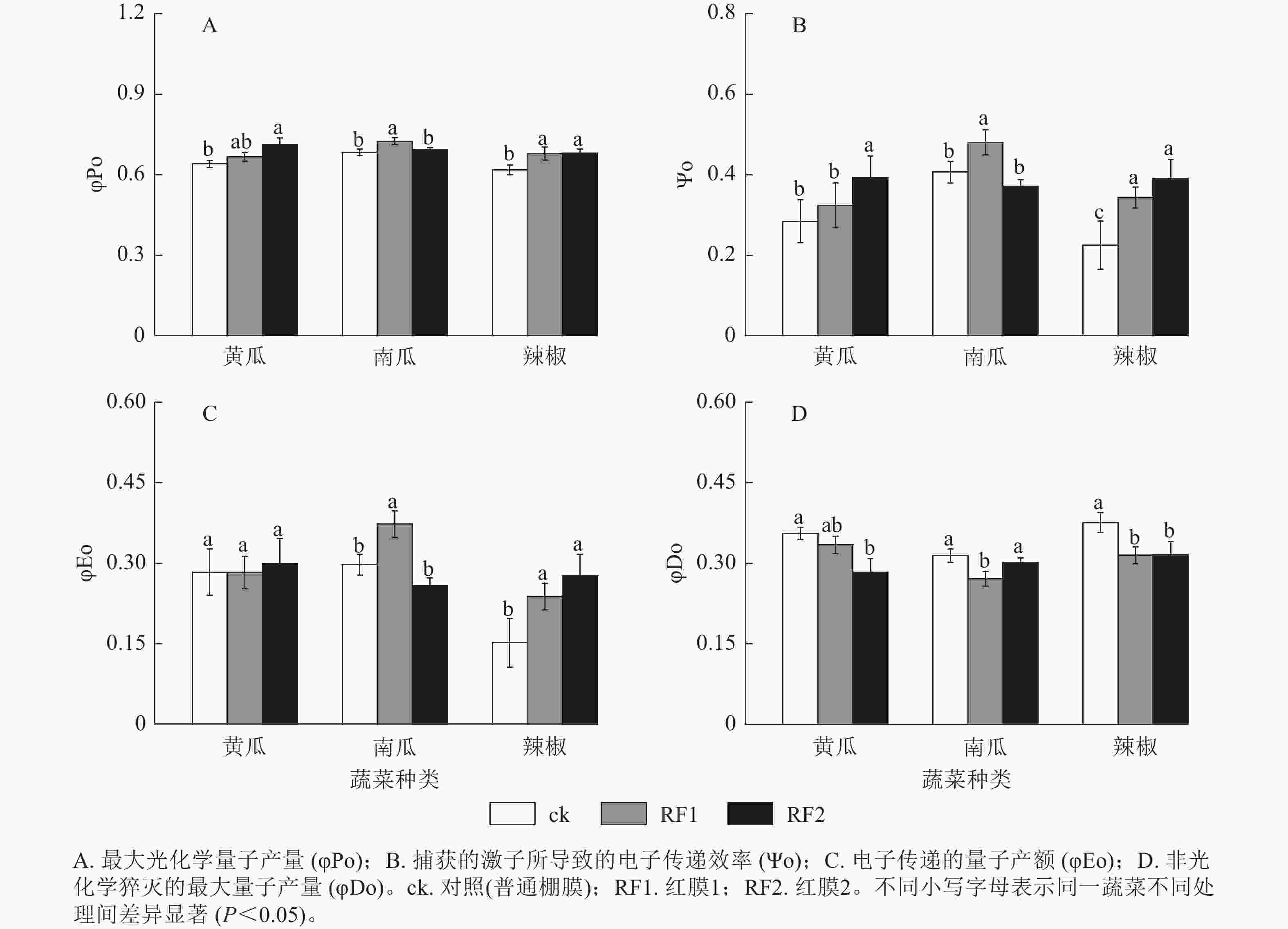
与对照相比,RF2红膜使黄瓜φPo显著提高了10.1% (P<0.05),RF1红膜使南瓜φPo显著提高了6.6% (P<0.05),RF1和RF2分别使辣椒φPo显著提高了10.0%和9.9% (P<0.05) (图2A)。RF1和RF2对3种蔬菜Ψo的影响与φPo相似(图2B)。与对照相比,RF1使南瓜φEo显著提高了25.6% (P<0.05),RF1和RF2分别使辣椒φEo显著提高了56.6%和81.6% (P<0.05) (图2C)。此外,RF1和RF2分别使南瓜和黄瓜φDo显著(P<0.05)降低,并均使辣椒φDo显著(P<0.05)降低(图2D)。
-
黄瓜在RF2大棚栽培时,果实中蔗糖、果糖、还原糖和总糖质量分数分别较对照显著增加了22.9%、44.4%、9.9%和14.7% (P<0.05)。与对照相比,RF1和RF2均不影响黄瓜中葡萄糖质量分数,但在RF1大棚栽培时葡萄糖质量分数却显著(P<0.05)高于RF2大棚栽培(表1)。
蔬菜种类 处理 质量分数/(mg·g−1) 蔗糖 葡萄糖 果糖 还原糖 总糖 黄瓜 ck 13.31±0.37 b 16.99±0.27 ab 6.15±0.25 b 23.14±0.43 b 36.45±0.49 b RF1 11.97±0.46 b 18.68±0.25 a 5.47±0.27 b 24.15±0.42 ab 36.12±0.73 b RF2 16.36±0.63 a 16.55±0.14 b 8.88±0.40 a 25.43±055 a 41.79±0.88 a 南瓜 ck 16.88±0.33 b 32.62±0.60 a 14.16±0.33 b 46.78±0.99 b 63.66±0.10 b RF1 17.90±0.48 ab 32.28±0.34 a 14.55±0.09 b 46.83±0.65 b 64.73±0.64 b RF2 19.49±0.53 a 34.16±0.23 a 16.18±0.28 a 50.34±0.50 a 69.83±0.53 a 辣椒 ck 10.41±0.46 b 20.76±0.44 a 11.37±0.57 a 32.13±0.74 a 42.54±0.90 a RF1 11.77±0.69 ab 20.09±0.39 a 11.48±0.35 a 31.57±0.96 a 43.34±1.47 a RF2 12.56±0.35 a 19.57±0.42 a 12.65±0.37 a 32.22±0.74 a 44.78±1.14 a 说明:ck. 对照(普通棚膜);RF1. 红膜1;RF2. 红膜2。不同小写字母表示同一蔬菜不同处理间差异显著(P<0.05)。 Table 1. Effects of 2 red films on sugar content in cucumber, squash and pepper fruits
南瓜在RF2大棚栽培时,果实中蔗糖、果糖、还原糖和总糖质量分数最高,分别较对照显著提高了15.5%、14.3%、7.6%和9.7% (P<0.05) (表1)。
与对照相比,RF2可显著(P<0.05)提高辣椒果实中蔗糖质量分数,然而RF1和RF2均不影响其他糖分积累(表1)。
-
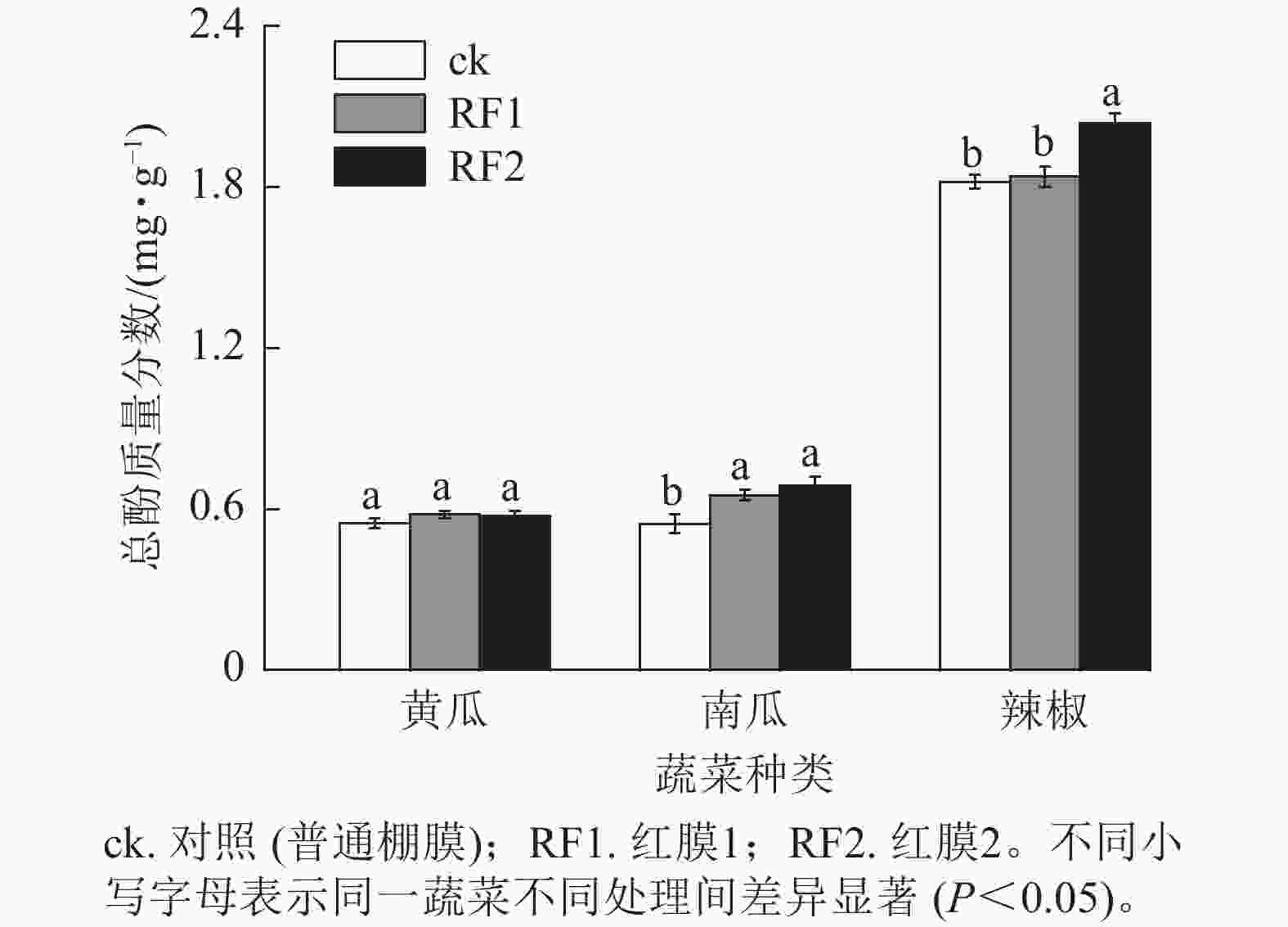
与对照相比,RF1和RF2大棚栽培时黄瓜果实中总酚质量分数无显著差异,而南瓜果实中总酚质量分数分别显著提高了19.6%和26.1% (P<0.05)。与对照相比,RF1不影响辣椒果实中总酚质量分数,但RF2则使辣椒总酚质量分数显著提高了12.0% (P<0.05) (图3)。
-
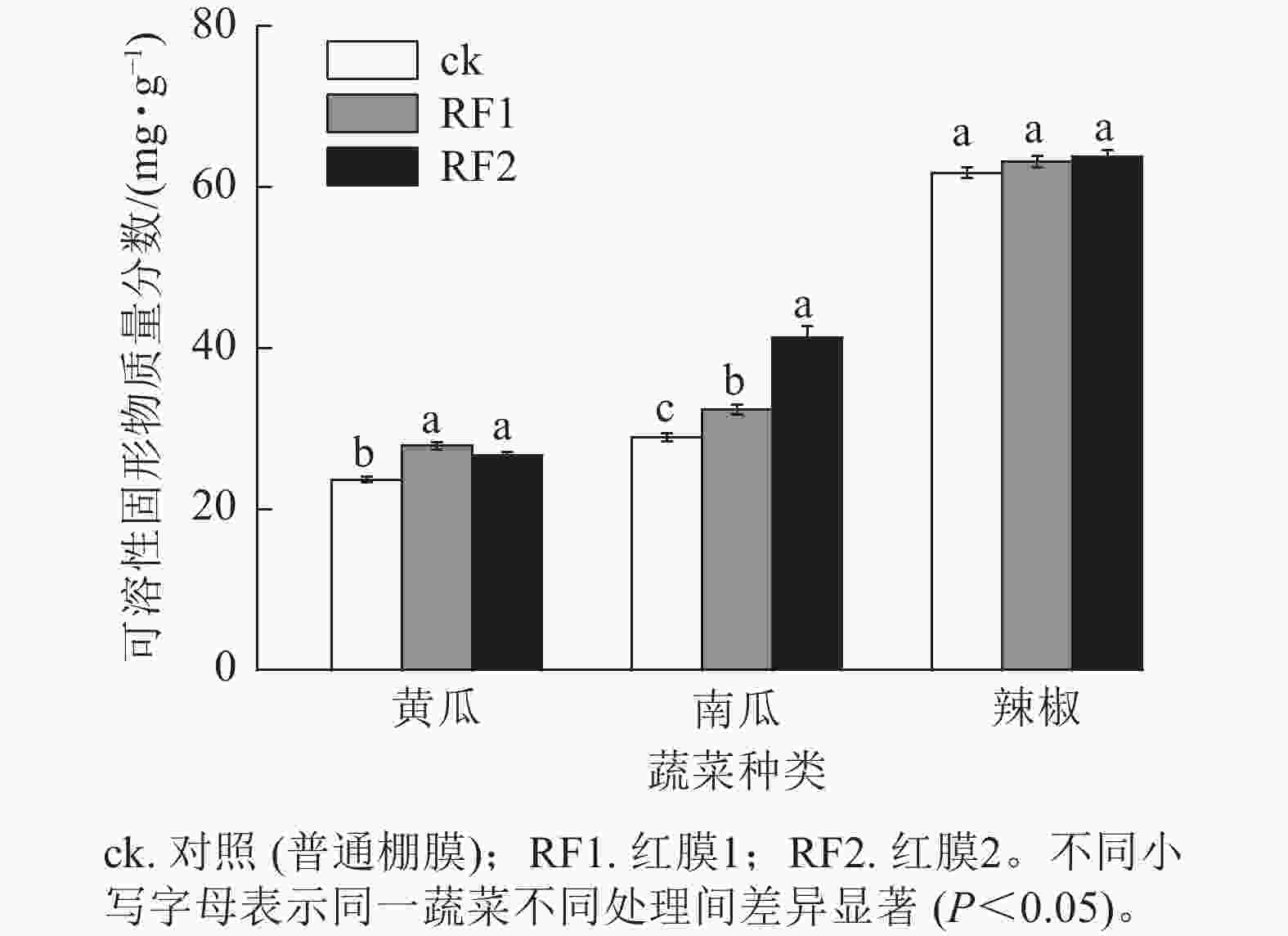
在RF1和RF2大棚栽培时,黄瓜果实中可溶性固形物质量分数分别较对照显著提高了14.9%和10.1% (P<0.05),南瓜果实中可溶性固形物质量分数分别显著提高了11.9%和42.7% (P<0.05)。与对照相比,RF1和RF2对辣椒果实中可溶性固形物质量分数均无显著影响(图4)。
-
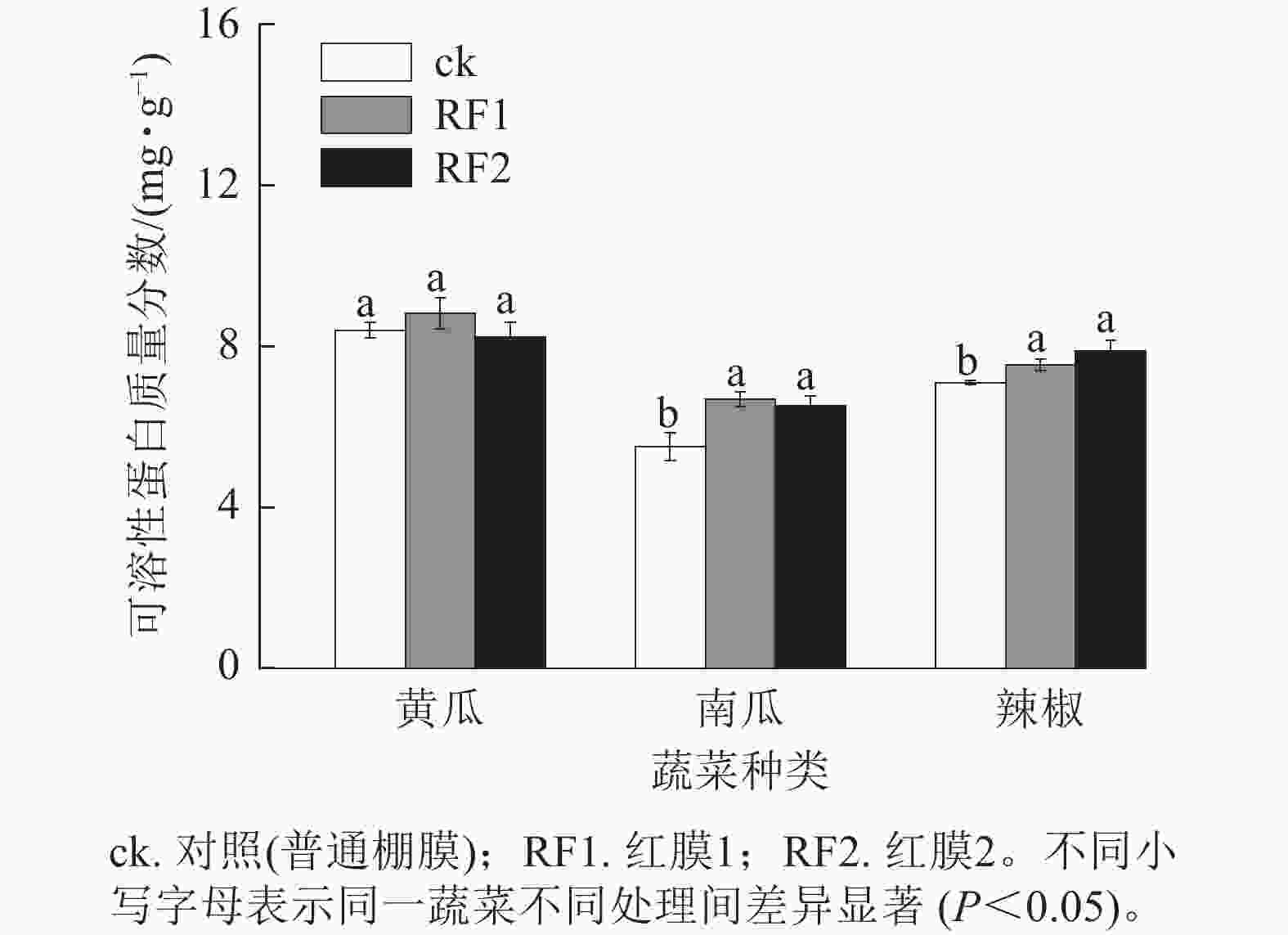
虽然RF1和RF2对黄瓜果实中可溶性蛋白质量分数均无显著影响,但却显著提高了南瓜和辣椒果实中可溶性蛋白质量分数(P<0.05)。与对照相比,南瓜果实中可溶性蛋白质量分数分别提高了21.4%和18.5%,辣椒果实中可溶性蛋白质量分数分别提高了6.2%和11.1% (图5)。
-
光合作用是植物最基本的物质与能量代谢过程,光合产物是果蔬中糖、蛋白质等初生代谢产物以及总酚、可溶性固形物等风味物质的合成原料[19]。叶绿素荧光分析是一种快速、非侵入式和高灵敏度检测植物光合性能的方法,其荧光参数可反映植物对光能的吸收、传递与利用情况[3]。与白光相比,红光可提高番茄叶片光合电子传递效率,而绿光和黄光则呈现相反效应[27]。与蓝光相比,红光可提高茄子[11]与番茄[12]的光合速率。在红色转光膜处理后,甜椒Capsicum annuum var. grossum的净光合速率与气孔导度均明显提高,从而促进果实产量增加[28]。在不同颜色的棚膜中,红膜可提高草莓φPo和φEo,而紫膜则呈现相反的效应[3]。在光反应过程中,植物吸收光后未被利用的光能可通过放热形式进行散失(φDo)[3]。在红膜处理下,草莓φDo降至最低,而紫膜处理下则升至最高[3],这表明红膜可提高植株光能利用效率并降低热耗散。本研究2种红膜均可提高黄瓜、南瓜和辣椒的最大光化学量子产量、电子传递效率与量子产额,并降低热耗散,促进3种蔬菜的PSⅡ效率提高,与已有研究结果相似。其中,RF1和RF2分别对南瓜和黄瓜具有较好的促进效应,对辣椒具有相同的促进效应。
在不同光质中,红光不仅可提高樱桃番茄产量,还可提高果实中番茄红素与可溶性糖质量分数[15]。此外,红光还能提高豌豆芽苗菜、辣椒果实与芦笋Asparagus officinalis的可溶性糖质量分数,从而促进其品质提升[14, 16, 29]。在不同颜色的棚膜中,红膜可促进草莓果实中果糖、葡萄糖、蔗糖和总糖积累[19]。在本研究中,RF2显著提高了黄瓜和南瓜果实中蔗糖、果糖、还原糖和总糖质量分数,以及辣椒果实中蔗糖质量分数。糖是光合作用的主要产物,高光合速率可促进糖在果实中积累[19, 28]。RF2可不同程度提高3种蔬菜的光合性能,从而提高糖分在果实中积累,其积累差异可能与蔬菜品种有关[30]。在不同光质下,光合产物的分配存在一定差异,红光和紫光可分别促进光合产物向初生代谢和次生代谢进行分配[31]。在3种棚膜中,RF1具有较高的紫光和紫外光透过率。这可能促进了3种蔬菜在RF1下将较多的光合产物分配给次生代谢而非果实中糖分形成,因而糖分未明显增加。
在2种红膜处理下,RF1可显著提高南瓜果实中总酚质量分数、南瓜和辣椒果实中可溶性蛋白质量分数,RF2可显著提高南瓜和辣椒果实中总酚与可溶性蛋白质量分数,这表明红膜处理有利于促进以上2种蔬菜的营养品质提升。这与红膜促进草莓果实中可溶性蛋白积累[3]、红色转光膜提高黄瓜果实中游离氨基酸和可溶性蛋白质量分数[32]的研究结果相一致,可能因为红光通过诱导胞内Ca2+信号,促进氨基酸合成相关基因表达、上调蛋白质合成以及其他相关代谢途径基因表达[33−35]。与单一白光处理相比,白光+红光+蓝光处理可提高番茄果实中可溶性固形物质量分数[36]。在本研究中,RF1和RF2处理均能明显提高黄瓜和南瓜果实中可溶性固形物质量分数,促进2种蔬菜风味品质提升。
-
红膜RF1和RF2可不同程度提高黄瓜、南瓜和辣椒叶片中光合电子产生与传递,从而提高3种蔬菜光合性能。2种红膜均能明显促进3种蔬菜果实中可溶性蛋白积累,提高南瓜和黄瓜果实中可溶性固形物质量分数,促进南瓜和辣椒(RF2处理)果实中总酚积累。然而,RF2更有利于提高3种蔬菜果实中糖、南瓜可溶性固形物和辣椒总酚。因此,RF2更适宜于搭建设施大棚,从而促进果蔬品质提升。
Effects of red film on photosynthetic abilities and fruit qualities in 3 vegetables
doi: 10.11833/j.issn.2095-0756.20240552
- Received Date: 2024-09-25
- Accepted Date: 2024-12-30
- Rev Recd Date: 2024-12-12
- Publish Date: 2025-05-30
-
Key words:
- red film /
- fruit vegetable /
- photosynthetic ability /
- fruit quality
Abstract:
| Citation: | LI Luyao, ZHANG Jun, YING Xuebing, et al. Effects of red film on photosynthetic abilities and fruit qualities in 3 vegetables[J]. Journal of Zhejiang A&F University, 2025, 42(3): 564−571 doi: 10.11833/j.issn.2095-0756.20240552 |











 DownLoad:
DownLoad:



