-
菊花Chrysanthemum × morifolium是菊科Asteraceae菊属Chrysanthemum植物,是观赏植物中变异类型最丰富的花卉之一,在中国已有3 000多年的栽培历史[1−2] ,品种资源丰富,文化底蕴深厚。菊花是药食兼用功能型花卉[3−4],其药用价值最早记载于《神农本草经》[5],并被《中国药典》收录为散热清风、平肝名目的药材,也是国家卫生健康委员会认定的8种花源性食用药之一。此外,菊花还具有多种人体必需的氨基酸[6],广泛应用于功能性食品和饮料中[2, 7]。近年来的研究揭示了菊花具有抗氧化、抗炎抑菌等生物活性,其活性成分的鉴定与功能研究不断深入。鉴于其天然来源的安全性,解析菊花活性物质的合成调控机制并开发绿色合成技术具有重要的科学意义和应用价值。本研究系统总结了菊花中活性物质的种类、合成途径及其调控机制的研究进展,旨在为菊花活性成分开发、绿色高效合成和多功能新品种培育提供理论支持,推动菊花在观赏、食用和药用领域的多元化发展,提升其经济与社会效益。
-
类黄酮、酚酸和萜类化合物是菊花中主要的活性物质类型,也是评价其质量的关键指标。随着高效液相色谱法(HPLC)、液相色谱-质谱联用技术(LC-MS)和核磁共振波谱法(NMR)等高灵敏度、高准确性植物代谢组学分析技术的广泛应用,菊花中活性物质的种类得以更全面且精确地解析 [8]。目前已知的菊花活性物质见表1。
分类 名称 测定方法 测定部位 参考文献 类黄酮 芹菜素-7-葡萄糖苷apigenin-7-O-glucoside UF-LC-MS、UPLC 干花序 [18−21] 木犀草素luteolin NMR、UPLC-MS/MS、HPLC-MIPSPE 干花序 [22−25] 木犀草苷luteoloside UPLC-ESI-Q-TOF-MS/MS、UF-LC-MS、UPLC-MS/MS 鲜花序、干花序 [8, 19−20, 23, 26] 木犀草素-7-葡萄糖醛酸苷luteolin-7-O-glucuronide LC-MS、UF-LC-MS、UPLC-MS/MS 鲜花序、干花序 [8, 19−20, 26] 橙皮素hesperetin LC-MS、NMR 鲜花序、干花序 [8, 24] 山柰酚kaempferol HPLC-MIPSPE 干花序 [24] 杨梅素myricetin HPLC-MIPSPE 干花序 [24] 芹菜素apigenin LC-MS、NMR、UF-LC-MS 鲜花序、茎、
干花序[8, 22−23] 槲皮素quercetin UF-LC-MS、NMR 干花序、茎 [8, 22] 芦丁quercetin 3-O-rutinoside LC-MS 鲜花序 [8] 金合欢素acacetin UF-LC-MS、NMR 干花序、茎 [8, 22] 圣草酚eriodictyol NMR 茎 [22] 异鼠李素isorhamnetin HPLC-MIPSPE 干花序 [25] 樱黄素prunetin HPLC-Q-TOF-MS/MS 干花序 [21] 酚酸 1,4-O-二咖啡酰奎宁酸1,4-dicaffeoylquinic acid LC-MS、NMR、UPLC 干花序、茎 [8, 19, 22] 1,3-O-二咖啡酰奎宁酸1,3-dicaffeoylquinic acid UPLC-MS/MS 干花序 [26] 1,5-O-二咖啡酰奎宁酸1,5-dicaffeoylquinic acid LC-MS、NMR、UF-LC-MS 干花序、茎 [8, 20, 22] 3,5-O-二咖啡酰奎宁酸3,5-dicaffeoylquinic acid LC-MS、NMR、UF-LC-MS、UF-LC-MS 干花序、茎 [8, 20, 22−23] 4,5-O-二咖啡酰奎宁酸4,5-dicaffeoylquinic acid LC-MS、UPLC 干花序 [8, 19] 绿原酸chlorogenic acid LC-MS、NMR、UF-LC-MS、UPLC-MS/MS 干花序序、茎 [8, 20, 22−23, 26] 咖啡酸affeic acid LC-MS 干花序 [8] 没食子酸gallic acid NMR 茎 [24] 萜类 β-榄香烯(–)-β-elemene GC-MS 干花序 [17] (E)-β-金合欢烯cis-β-farnesene GC-MS 干花序 [17] β-半水芹烯(–)-β-sesquiphellandrene GC-MS 干花序 [17] (+)-喇叭烯(+)-ledene GC-MS 干花序 [17] 桉叶油素1,8-cineole GC-MS 鲜花序 [16] 樟脑camphor GC-MS 鲜花序 [16] 丁香酚eugenol GC-MS 鲜花序 [16] β-石竹烯β-caryophyllene GC-MS 鲜花序 [16] α-姜烯α-zingiberene GC-MS 鲜花序 [16] β-蒎烯β-curcumene GC-MS 鲜花序 [16] 石竹烯氧化物caryophyllene oxide GC-MS 鲜花序 [16] α-没药醇α-bisabolol GC-MS 鲜花序 [16] 香芹酮carvone NMR 茎 [24] 说明:UF-LC-MS. 超滤液相色谱-质谱联用技术;UPLC. 超高效液相色谱法;NMR.核磁共振波谱法;UPLC-MS/MS. 超高效液相色谱-串联质谱联用技术;HPLC-MIPSPE. 高效液相色谱-分子印迹固相萃取在线联用技术;UPLC-ESI-Q-TOF-MS/MS. 超高效液相色谱-电喷雾离子源-四级杆-飞行时间串联质谱联用技术。LC-MS. 液相色谱-质谱联用技术;GC-MS.气相色谱-质谱联用技术。 Table 1. Known bioactive compounds in C. morifilium
-
类黄酮是一类由植物次级代谢产生的多酚类化合物,广泛分布于水果、蔬菜和其他粮食作物中,对植物抵御生物和非生物胁迫至关重要[9] 。在菊花中,类黄酮是活性物质的主要成分,已鉴定出黄烷酮、黄酮类、黄酮醇及其苷类和衍生物等类黄酮不同亚类[8, 10] ,包括木犀草素、芹菜素、金合欢素、异鼠李素、橙皮苷、香叶木素、山柰酚及其衍生物等代表性化合物[11−14]。
-
菊花中酚酸类物质含量较低,却是其药理作用的关键成分。绿原酸(CGA)、3,5-O-二咖啡酰奎宁酸被《中国药典》列为质量控制指标,而4,5-O-二咖啡酰基奎宁酸则是菊花和野菊中常见的酚酸类物质[7, 15]。此外,‘杭菊’‘Hangju’、‘贡菊’‘Gongju’、‘怀菊’‘Huaiju’、‘福白菊’‘Fubaiju’等品种均含多种绿原酸异构体[14]。L-(−)-3-苯基乳酸和L-苹果酸等有机酸类是‘皇菊’‘Huangju’、‘金丝皇菊’‘Jinshihuangju’等菊花茶呈现苦味和鲜味的原因[13]。
-
萜类化合物是菊花中的主要挥发性成分,也是茶菊、精油风味的关键因素。菊花中萜类成分以倍半萜类为主,单萜较少,主要化合物包括樟脑、桉油精、α-姜黄烯、α-姜烯、β-榄香烯、红没药醇、β-倍半水芹烯、(+)-喇叭烯、(E)-β-金合欢烯等[16−17]。除花序外,菊花地下部位也富含萜类物质,通过气相色谱-质谱联用技术(GC-MS)对26个菊花品种根提取物的鉴定发现:‘中阳菊’‘Zhongyangju’等茶菊品种根系的总萜含量普遍高于‘中山梦庄’‘Zhongshanmengzhuang’等地被菊和‘白乒乓’‘Baipingpang’等切花菊品种 [3]。
-
菊花作为传统草药,源于天然植物,具有低成瘾性和高安全性[27],在抗炎抑菌、抗肿瘤、降血脂、增强免疫活性等方面表现出显著功效[28−36]。菊花花序中的多种黄酮类化合物,如圣草酚-7-O-葡萄糖苷、黄酮苷、芹菜素-7-O-葡萄糖苷、香叶木素-7-O-葡萄糖苷等被证实是透明质酸酶(HAase)的强效抑制剂,它们能显著抑制小鼠和人类巨噬细胞中诱导型一氧化氮合成酶(iNOS)的活性以及白细胞介素-1β (IL-1β)的表达,进而显著降低一氧化氮(NO)和白细胞介素-6 (IL-6)的水平[19]。此外,菊花中山奈酚、4-羟基苯甲酸和芹菜素与黄嘌呤氧化酶(XO)抑制活性密切相关,且这3种成分之间存在相加作用[24]。
菊花精油富含活性物质,具有优异的抗氧化活性和感官改善性能[37−39],是天然防腐剂的丰富来源[40],可广泛应用于食品添加剂和医药领域[17]。如添加菊花精油可显著抑制葵花Helianthus annuus籽油中油脂变质指标的上升[41]。此外,菊花与其他天然中草药混合时能产生协同作用,如菊花与黄芩Scutellaria baicalensis的热水提取物表现出更强的抗氧化活性[12]。
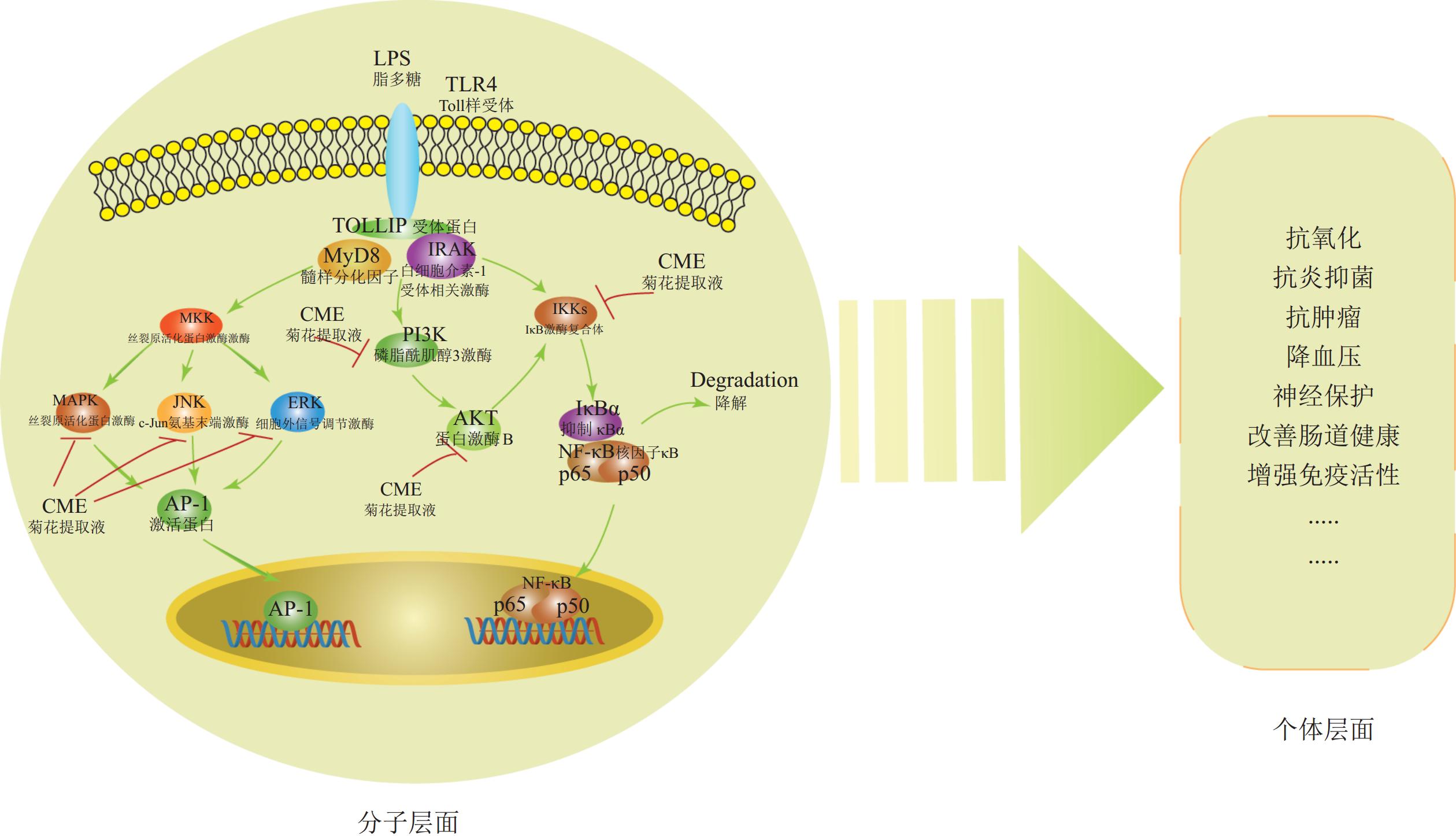
菊花活性物质的功效分子机制已部分阐明。这些物质通过调控丝裂原活化蛋白激酶(MAPK)、磷脂酰肌醇-3-激酶(PI3K/ Akt)和核因子-κB (NF-κB)信号通路,减少NO生成,抑制iNOS、肿瘤坏死因子-α(TNF-α)、IL-1β和IL-6的转录活性,同时抑制IκB激酶(IKK)、磷脂酰肌醇3-激酶(PI3K)的磷酸化,下调细胞外信号调节激酶(ERK)、 c-Jun氨基末端激酶(JNK)、p38丝裂原活化蛋白激酶(p38)、p50蛋白(p50)和p65蛋白(p65)的表达,从而发挥抗炎抗氧化等作用[26](图1)。此外,菊花的酪氨酸酶活性抑制能力(即抗氧化能力)与类黄酮和绿原酸等酚酸含量呈显著正相关[42]。
-
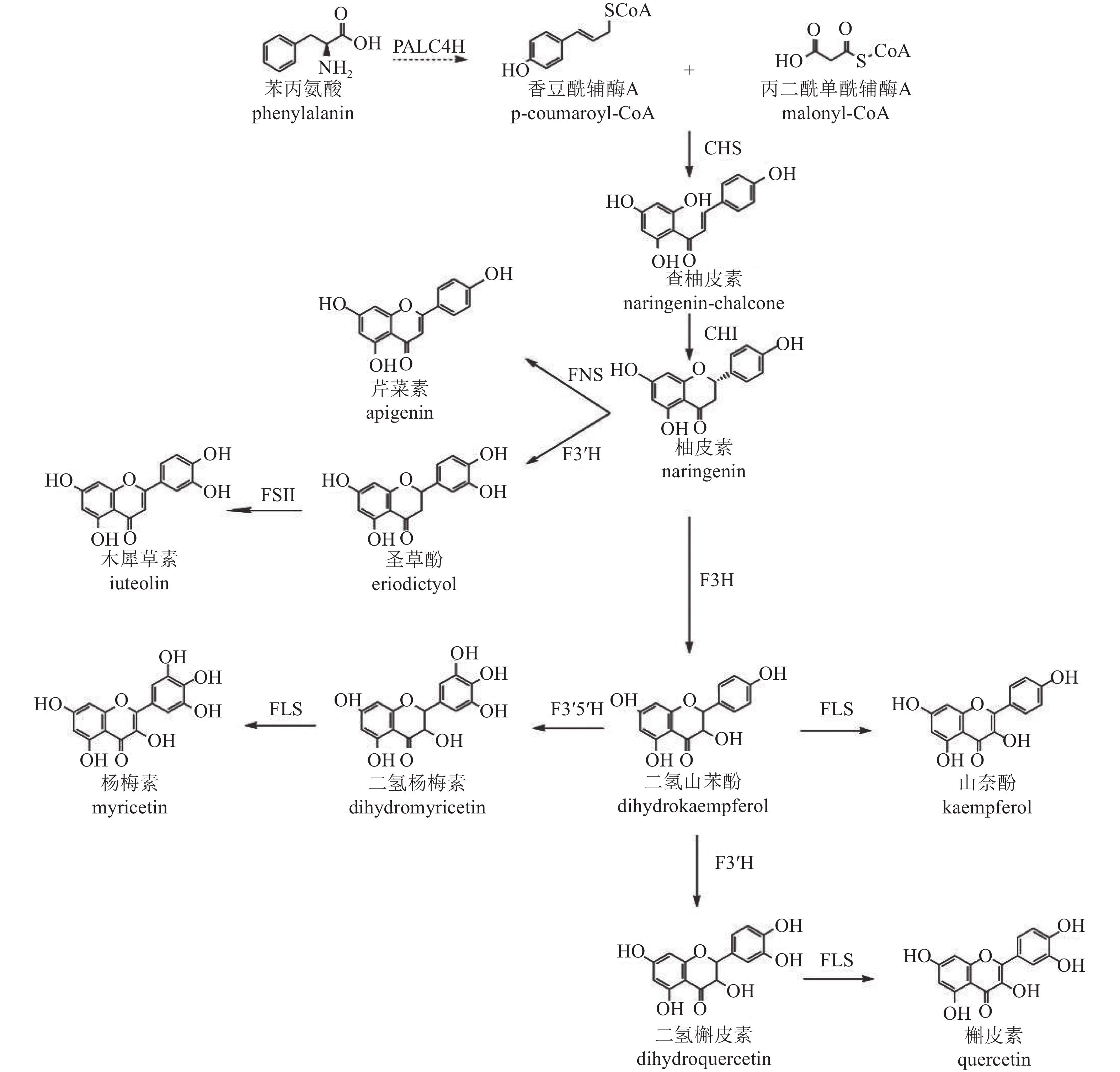
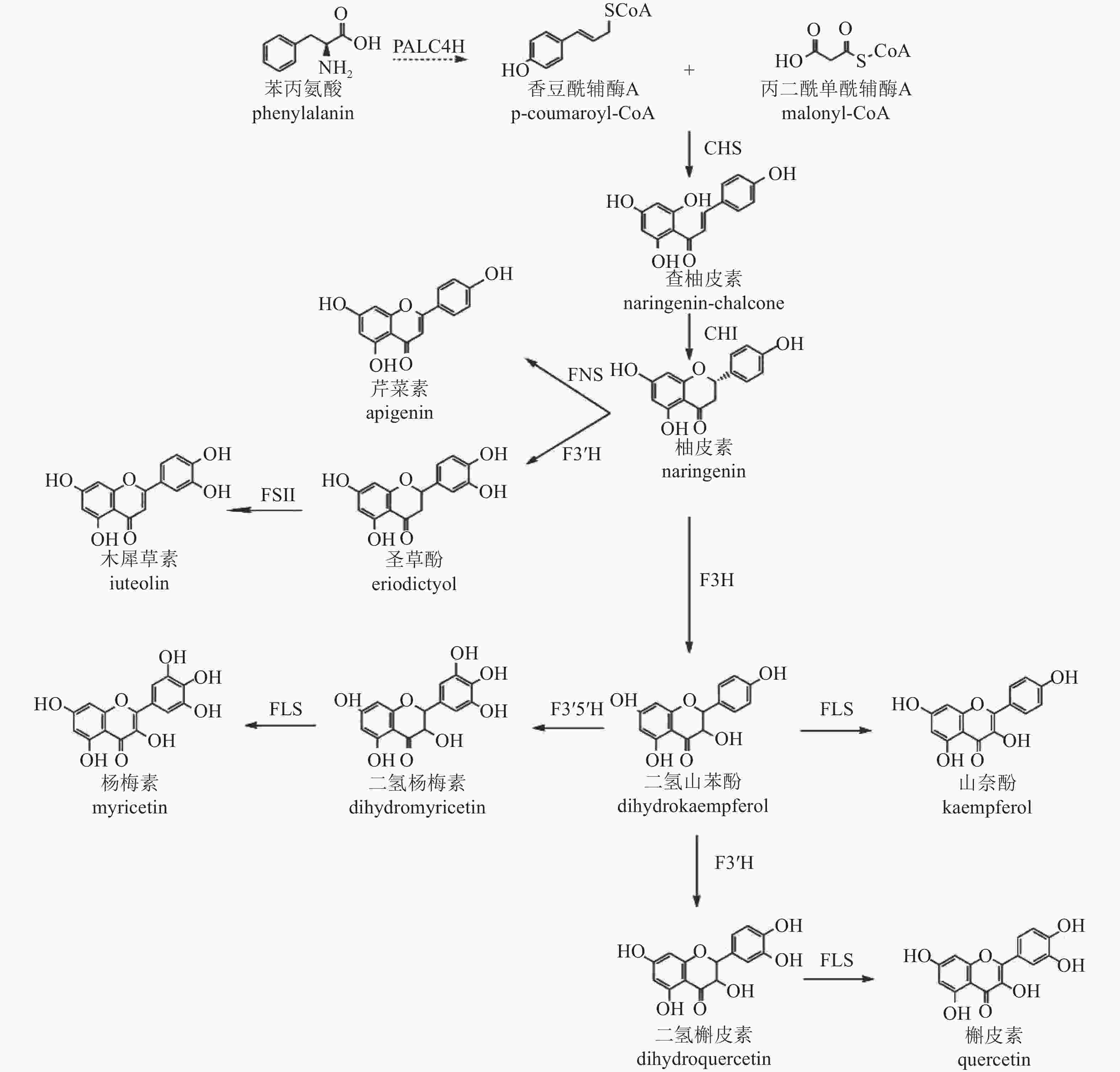
类黄酮化合物是植物中重要的次生代谢产物,其生物合成途径已较为明确[43],以香豆酰辅酶A (pCoA)为起始,在丙二酰单酰辅酶A (malonyl-CoA)参与下,经查尔酮酶(CHS)催化形成查柚皮素,并在查尔酮异构酶(CHI)进一步催化变构下形成类黄酮亚群常见的中间体柚皮素,再以柚皮素为基本骨架形成黄酮、黄烷酮、黄酮醇等其他类型化合物(图2)。根据基因在合成途径的位置,可将相关基因分为早期和晚期生物合成基因。早期生物合成基因(EBGs),包括查尔酮合成酶(CHS)、查尔酮异构酶(CHI)、二氢黄酮-3-氢化酶(F3H)、黄酮类-3'-氢化酶(F3'H)、黄酮醇合成酶(FLS),催化黄酮醇的生物合成,而晚期生物合成基因(LBGs)、二氢黄酮醇4-还原酶(DFR)、花青素合成酶(ANS)和花青素还原酶(ANR)等则促进花青素和原花青素的生物合成[44]。此外,其他基因如黄酮合成酶(FNS)和异黄酮合成酶(IFS)也分别催化黄酮和异黄酮的合成[45−46]。
转录组学分析表明:差异表达基因显著富集在类黄酮、不饱和脂肪酸、苯丙素和类胡萝卜素的生物合成途径[47],菊花中参与类黄酮生物合成的关键基因有苯丙氨酸裂解酶(PAL)、肉桂酸-4-氢化酶(C4H)、4-香豆酸辅酶A连接酶(4CL)、CHS、CHI、F3H、F3'H、FLS、FNS Ⅱ、二氢黄酮醇还原酶(DFR)和ANS。对木犀草素和槲皮素合成相关基因家族分析显示,存在5个CmCHS、3个CmCHI、1个CmF3H、1个CmF3'H、1个CmFNS Ⅱ和2个CmFLS基因异构体。在药用菊花中未发现与催化蓝花青素底物黄酮类-3'-5'-氢化酶(F3'5'H)相似的序列,这与缺乏蓝色药用菊花品种的现象一致。此外,ANR和无色花青素还原酶基因(LAR)也未在数据库中被鉴定,进一步解释了药用菊花中原花青素的缺失[48]。
菊花中CHS、CHI、F3'H等类黄酮合成基因已被成功克隆并鉴定功能。CHS、CHI作为植物聚酮合酶超家族成员,在类黄酮合成途径中起关键作用,影响黄酮类代谢产物的积累[49−50],且表达具有显著组织特异性[48]。F3'H基因编码的酶催化黄酮类化合物B环3-O位置的羟基化[51−52],是下游化合物合成的关键酶。基因结构分析显示:‘贡菊’和‘滁菊’‘Chuju’的F3'H基因第2内含子中含有与转录密切相关的基序,表明内含子可能参与了药菊F3'H基因的表达调控[53]。
-
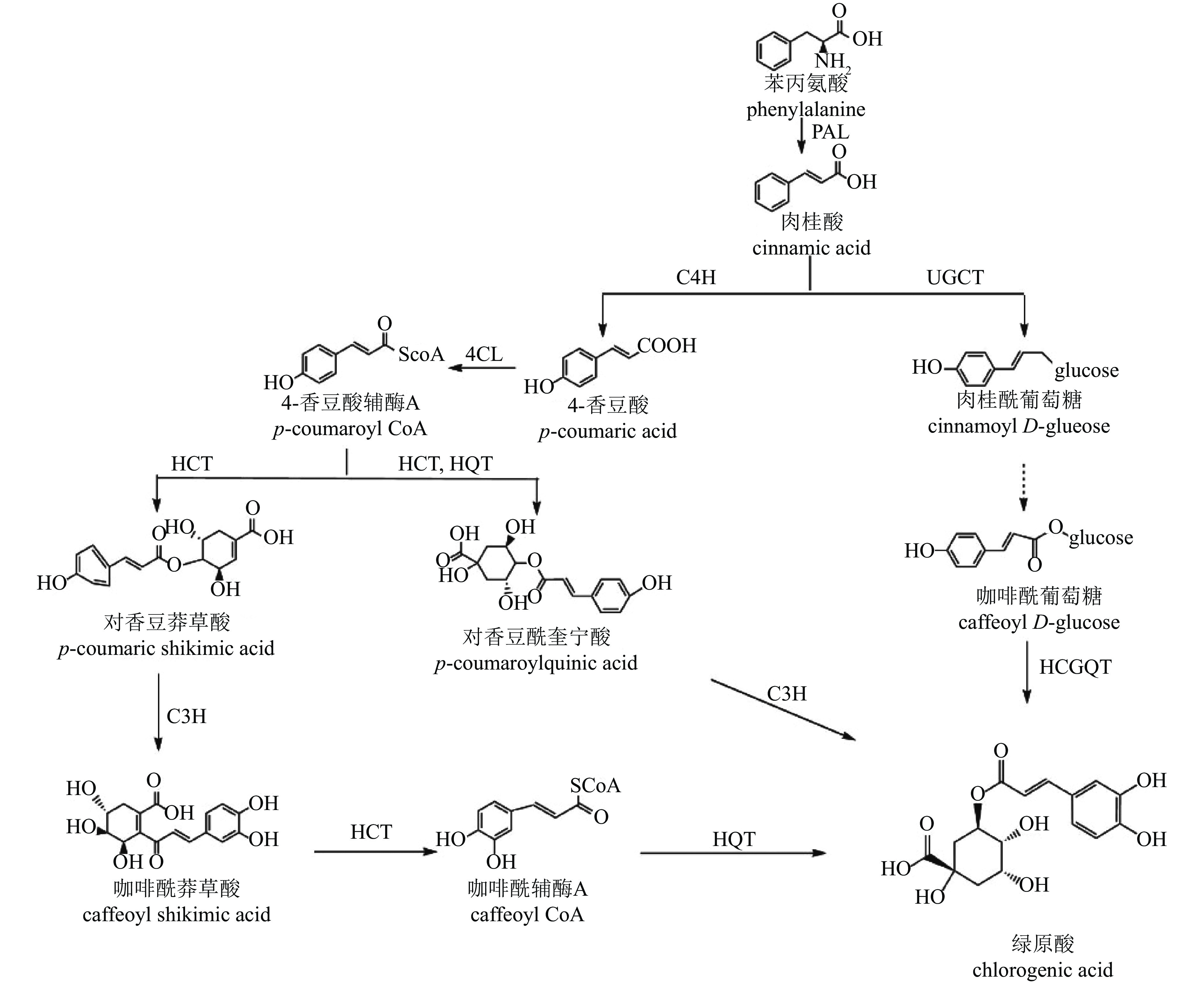
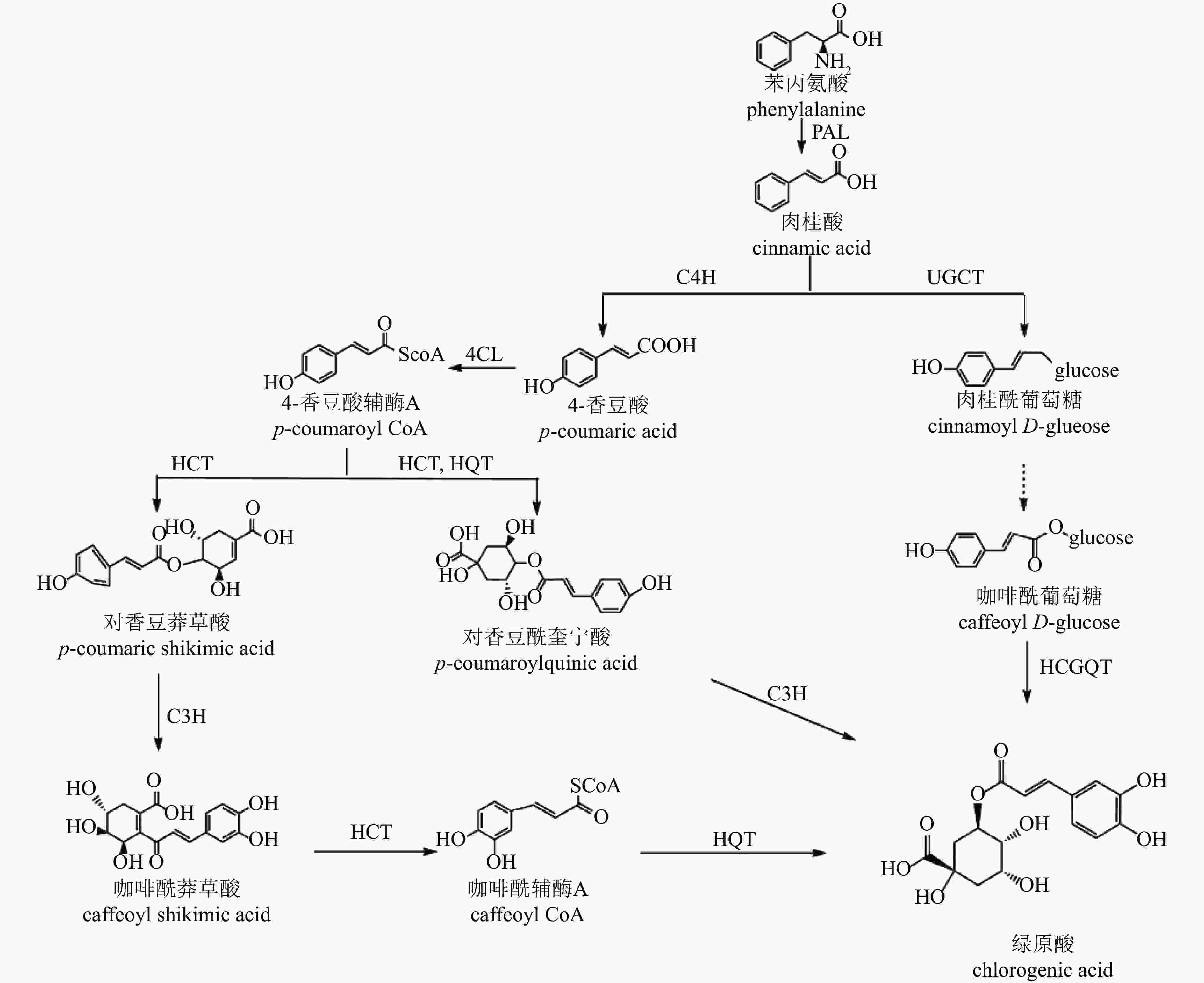
CGA的生物合成发生在植物次级代谢的苯丙素途径下游,目前已提出3种生物合成途径[54](图3):肉桂酸经肉桂酸4-羟化酶(C4H)催化生成中间产物4-香豆酸辅酶A(4-CL),在途径1中,该中间产物经奎宁酸羟基肉桂酰转移酶(HCT)、对香豆酸-3-羟化酶(C3H)、羟基肉桂酰辅酶A-奎宁酸羟基肉桂酰转移酶(HQT)等酶的连续作用转化为CGA;在途径2中,中间产物先形成对香豆酰奎宁酸(pCoQA),再由C3H催化生成CGA;在途径3中,肉桂酸经肉桂酸葡萄糖转移酶(UGCT)作用形成肉桂酰葡萄糖,进一步转化为咖啡酰葡萄糖,最终在奎宁酸羟基肉桂酰基转移酶(HCGQT)催化下形成CGA。
菊花中CGA合成研究较少。目前已有研究鉴定出13个HCT、2个HQT、2个C3H和3个UGCT基因与CGA合成有关[47]。
-
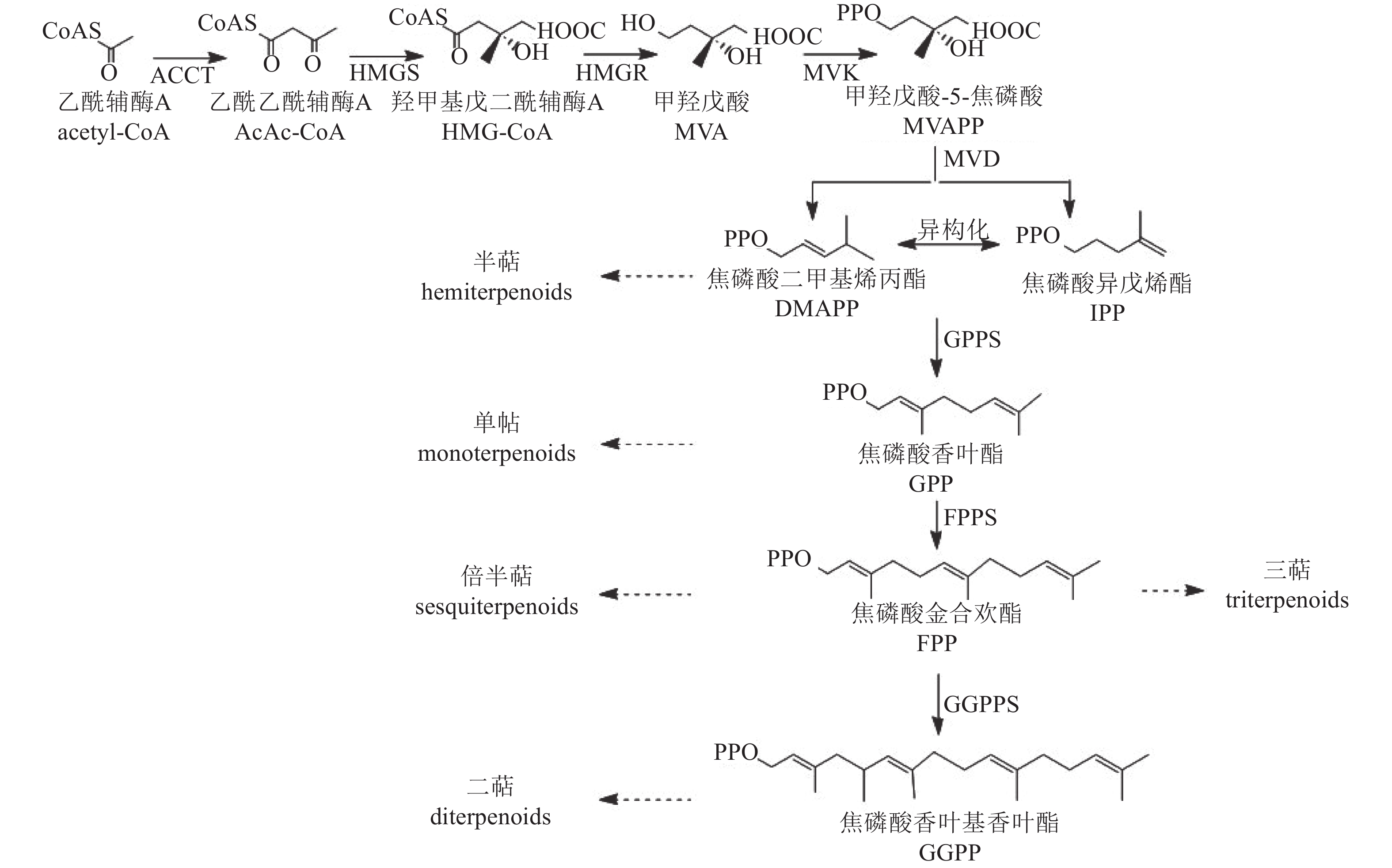
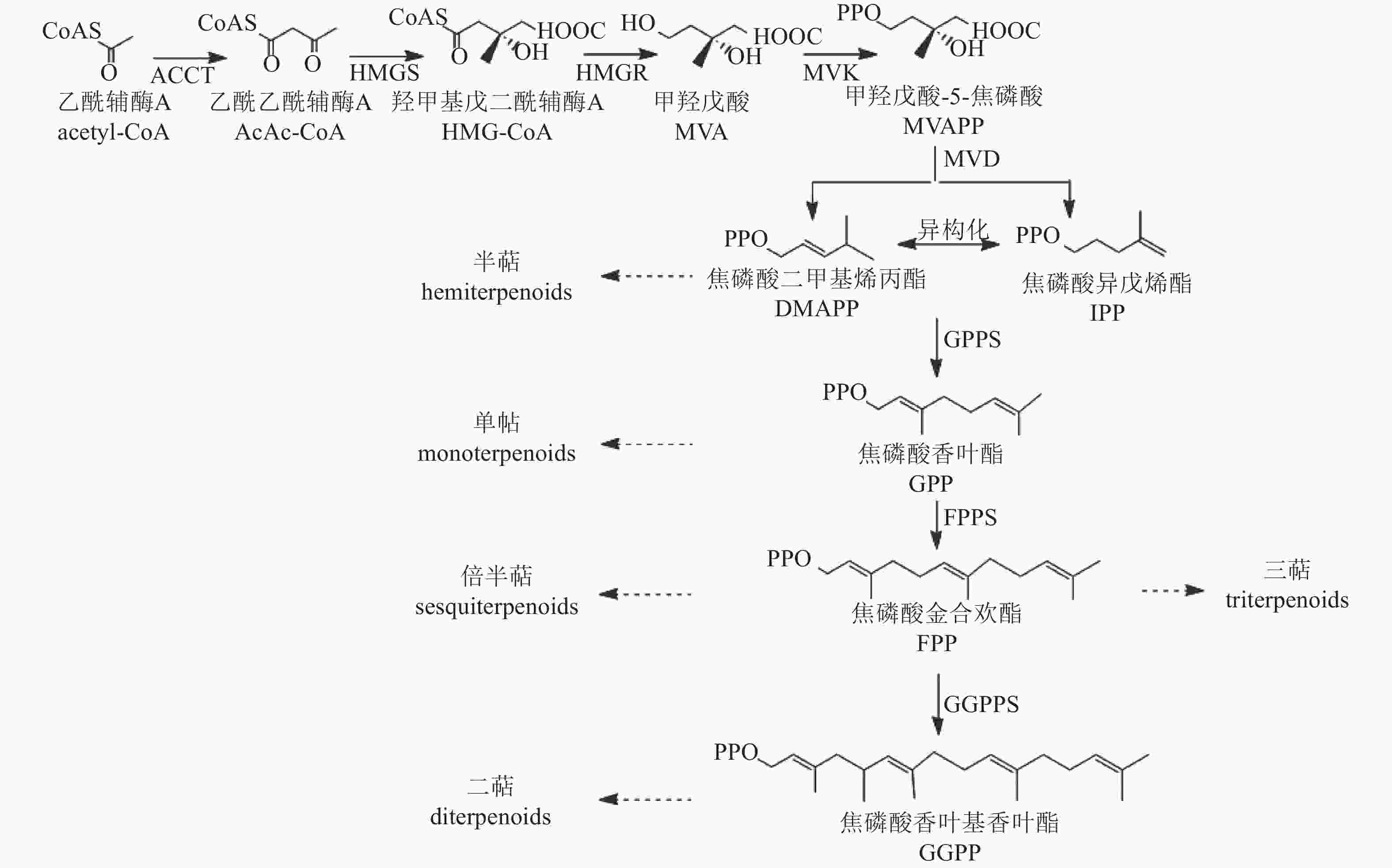
植物中萜类化合物主要经甲羟戊酸途径(MVA)合成[55](图4)。该途径从乙酰辅酶A缩合生成乙酰乙酰辅酶A开始,经羟醛缩合反应形成β-羟基-β-甲基戊二酸单酰辅酶A(HMG-GoA),随后生成中间体甲羟戊酸(MVA)。MVA经激酶催化形成甲羟戊酸-5-焦磷酸(MVAPP),再转化为焦磷酸异戊烯酯(IPP),IPP可异构化为焦磷酸二甲基烯丙酯(DMAPP)。最终,IPP和DMAPP在酶的作用下形成单萜、半倍萜、倍半萜、三萜等萜类化合物。
萜类化合物的多样性主要由萜类合成酶基因(TSs)驱动,包括TIDSs、SQSs、TPSs和TCCs。研究发现:TPS基因在菊属菊花脑C. nankingense基因组中显著扩增[56]。在‘滁菊’中鉴定出8个TPS基因,其编码蛋白均含有保守的二价阳离子结合域(DDXXD)和第二金属离子结合基序(NSE/DTE),这些结构是辅助因子Mg2+或Mn2+结合所必需的,其中,CmCJTPS5和CmCJTPS8被认为是菊花产生单半萜和倍半萜的关键基因。此外,CmCJTPS基因的表达水平与花香萜类挥发物的积累模式密切相关,萜类物质主要在完全开花前的盘状小花和叶根花托中合成[57]。
-
转录因子是一类能与基因5′端上游特定序列特异性结合、调控下游靶基因表达水平的蛋白质,在植物次生代谢中起关键作用[58]。目前已鉴定出多个转录因子家族参与植物次生代谢调控,其中MYB和bHLH家族研究最为深入。
MYB是植物转录因子家族的重要成员,其结构包含2个区域:N端高度保守,C端可变。这种特征赋予MYB蛋白多样化的功能[59]。MYB有3个亚类:MYB1R、R2R3-MYB和MYB3R,其中R2R3s是最大的亚类[60],主要来自SG4、SG5、SG6和SG7亚家族[61]。部分MYB参与调控开花时间、花药和花粉发育[62−63],例如在菊花‘金巴’‘Jinba’中,CmMYB2过表达的植株开花早于野生型,而CmMYB2表达受抑制的植株则开花延迟[64]。此外,一些MYB参与类黄酮的生物合成调控[65],转录分析显示菊花中CHS、CHI、F3H、F3'H和DRF的表达均受CmMYB8转基因抑制[66],在菊花‘粉色安娜斯塔’‘Anastasia Pink’中,CmMYB9a正向调节CmCHS、CmDFR和CmFNS,同时抑制CmFLS的表达,促进花青素和其他类黄酮积累,影响花色形成[67]。
bHLH转录因子的N端含有DNA结合的基本区域,C端含有HLH结构域,可调控植物的生长发育、次生代谢、抗逆性和信号转导[68],在活性物质合成尤其是花青素代谢中发挥重要作用。如丹参Salvia miltiorrhiza基因组中共鉴定出127个bHLH转录因子基因,其中7种可能参与丹参酮生物合成调控 [69],通过RNA干扰(RNAi)抑制丹参毛状根中SmbHLH92的表达,可显著增加酚酸和丹参酮积累[68]。在油菜Brassica napus籽中,BnbHLH92a通过抑制花青素和原花青素生物合成基因的表达,降低花青素积累,影响种子颜色[70]。酵母杂交实验表明:菊花中的CmbHLH2可直接结合CmDFR启动子,并与CmbHLH2和CmMYB6之间发生蛋白-蛋白相互作用,共同调控菊花花青素合成[71]。
AP2/ERF也是一个重要的转录因子大家族,通过结合靶基因启动子中的特定顺式作用元件调控基因表达,参与多种生理、发育和应激相关反应[72],包括AP2、ERF、RAV、Soloist等4个亚家族。染色质免疫沉淀(ChIP)和双萤光素酶(Dual-Luc)实验表明:烟草Nicotiana tabacum中的NtERF4a可结合NtPAL1和NtPAL2启动子中的GCC盒,激活其转录,促进烟草叶片中绿原酸和黄酮类化合物的积累[73]。HRE2-like作为AP2/ERF家族成员,在‘金巴’中调控类黄酮合成,CmCHS、CmCHI、CmF3'H在CmHRE2-like植株中下调,而在CmHRE2-like-SRDX植株上调,导致CmHRE2-like-SRDX系总黄酮含量显著增加[74]。
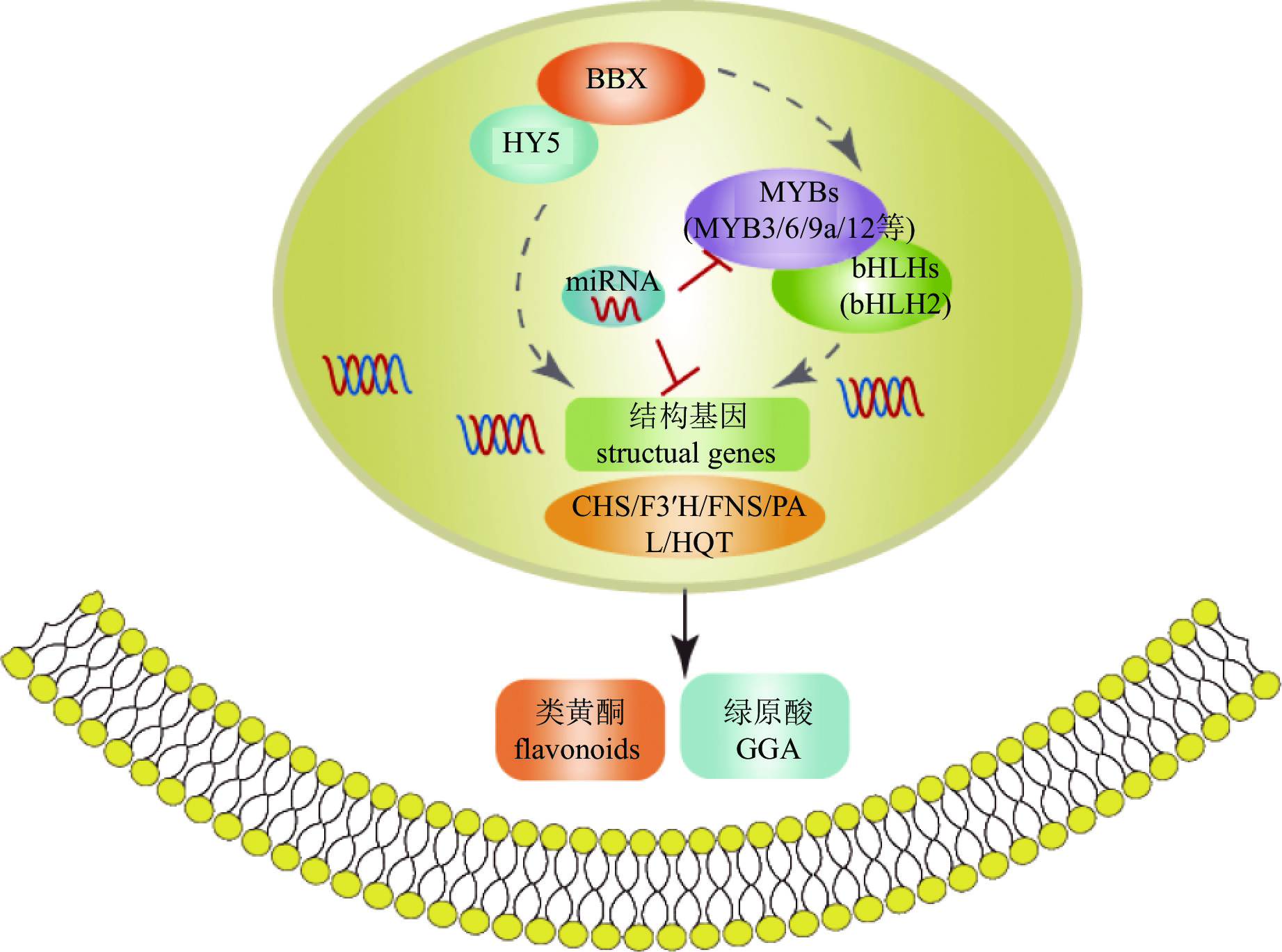
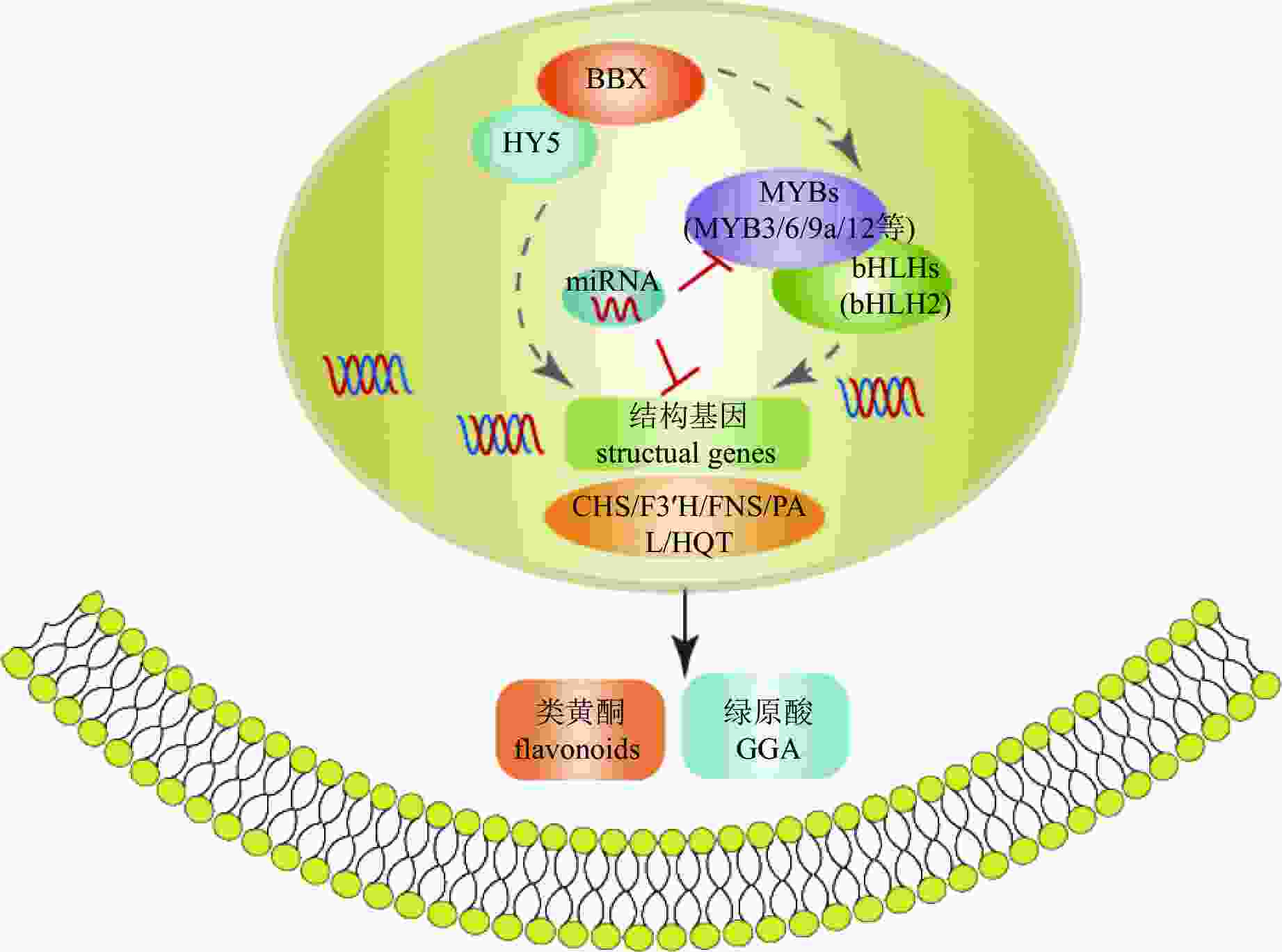
转录因子对活性物质合成的调控网络复杂且多层次,既可单独作用,也可通过互作调控。如菊花中BBX-HY5模块可能通过2种不同方式参与调控次生代谢(图5):一是直接调控CmHQT、CmCHS1/2、CmFNS、CmF3'H和CmPAL的转录水平;二是通过上调CmMYB3、CmMYB6和CmMYB16的表达水平,正向调节结构基因[75]。
-
miRNAs是一类广泛存在的内源性非编码小RNA分子,通过与下游靶向基因和转录因子结合参与调控多项生物学过程[76]。如芸薹Brassica rapa及其亲本miRNA表达谱分析发现有204个差异表达的miRNA,其中184个在芸薹中非加性表达,多组学数据表明miR858a和miR157a可能调控类黄酮合成[77]。有关菊花miRNA的研究相对有限,已鉴定出与HD-ZIP、DOF、SBF-like和Trihelix等转录因子家族相关的miRNA [78−81],并对其功能进行了初步分析。YANG等[47]检测到菊花中38个家族的169个miRNAs,其中118个miRNAs靶向1 954个基因,6种miRNA靶向5个活性成分生物合成调控相关基因,5个miRNAs通过靶向MYB-bHLH复合体相关转录因子,参与类黄酮和绿原酸的合成。
-
目前,植物活性物质合成过程中有关基因酶的克隆鉴定以及异源表达都取得了突破性进展。在天然次生代谢产物中,类黄酮化合物的结构及生物合成途径是目前研究最为清楚的途径之一,菊花中的活性物质研究也集中在类黄酮,包括木犀草素、芹菜素、金合欢素等,CmCHS、CmCHI、CmF3H、CmF3'H、CmFNS、CmFLS、Cm4CL、CmUGT是菊花类黄酮合成的关键基因,而TPS基因家族控制萜类合成酶(TSs)的合成,是菊花产生单半萜和倍半萜的决定因素,相比之下,绿原酸合成机制研究较少,有待进一步探索。
传统菊花活性物质提取是从植物中直接提取,受资源、季节、气候等影响较大,且天然含量低、化学合成困难,难以满足市场需求。因此,绿色高效的生物合成是未来获取菊花活性物质的重要途径,也是大健康领域研究热点。然而活性物质合成是一个多层次、立体的复杂网络,目前对其合成途径的总体调控以及次生代谢途径之间的协调机制等知之甚少,因此解析菊花活性物质的结构、合成途径及其调控网络,从不同侧面对活性物质合成过程及其调控加以整合分析和设计,是未来菊花活性物质利用的重点任务。
Research progress on the types, biosynthetic pathways, and regulatory mechanisms of bioactive compounds in Chrysanthemum × morifolium
doi: 10.11833/j.issn.2095-0756.20240567
- Received Date: 2024-10-10
- Accepted Date: 2025-06-30
- Rev Recd Date: 2025-04-14
- Available Online: 2025-10-29
- Publish Date: 2025-10-20
-
Key words:
- Chrysanthemum × morifolium /
- bioactive compounds /
- biosynthetic pathways /
- regulatory mechanisms
Abstract: Chrysanthemum × morifolium, a plant with a long history of ornamental, edible, and medicinal uses, contains bioactive compounds with multiple functions, such as anti-inflammatory, antioxidant, and anti-tumor properties. This article reviews the types and mechanisms of action of bioactive compounds in C. × morifolium, summarizes their biosynthetic pathways, and elucidates the regulatory mechanisms governing their synthesis. Chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonoid 3'-hydroxylase (F3'H), flavone synthase (FNS), flavonol synthase (FLS), 4-coumarate-CoA ligase (4CL), and UDP-glycosyltransferase (UGT) have been identified as key players in the synthesis of bioactive compounds. The biosynthesis is hierarchically regulated: transcription factor families such as MYB, bHLH, and AP2/ERF, modulate structural gene expression by binding to specific promoter elements. Additionally, miRNAs affect the synthesis of these compounds by targeting crucial structural genes and transcription factors. Although many researches have been conducted on flavonoid structure and biosynthesis in C. × morifolium, studies on the synthesis mechanism of chlorogenic acid, an important pharmacological component, remain limited and require further exploration. Clarifying the global regulatory network of bioactive compound biosynthesis and the molecular crosstalk between secondary metabolic pathways will be crucial for developing sustainable and efficient synthesis strategies for C. × morifolium active compounds, thereby advancing the development of the C. × morifolium industry. [Ch, 5 fig. 1 tab. 81 ref.]
| Citation: | ZHAO Fan, HUANG Guolin, XIAO Xiaoling, et al. Research progress on the types, biosynthetic pathways, and regulatory mechanisms of bioactive compounds in Chrysanthemum × morifolium[J]. Journal of Zhejiang A&F University, 2025, 42(5): 922−933 doi: 10.11833/j.issn.2095-0756.20240567 |









 DownLoad:
DownLoad: