-
开花是植物生命周期中的重要发育阶段,受自身遗传和外界环境因素的影响[1]。FCA(FLOWERING LOCUS CA)参与拟南芥Arabidopsis thaliana开花调控,通过多腺苷酸化(polyadenylation)和介导FLC(FLOWERING LOCUS C)染色质的组蛋白去甲基化(demethylation)调控开花[2-4]。拟南芥的fca突变后会抑制开花促进因子FT(FLOWERING LOCUS T)和SOC1(SUPPRESSOR OF OVEREXPRESSION OF CONSTANS)的表达,fca突变体在不同光周期下均表现出晚花表型[5]。另外,FCA可以激活LFY(LEAFY)和AP1(APETALA1)的活性促进拟南芥开花[6]。将水稻Oryza sativa和巴西橡胶树Hevea brasiliensis的FCA基因转入拟南芥fca突变体,会导致晚花性状出现逆转和恢复[7-8]。由此可见,FCA在植物花期调控方面发挥着重要作用。环境温度影响植物开花时间。植物FCA基因是温敏途径(thermosensory pathway)中的重要基因,可响应温度变化调控植物的花芽分化[9]。与16 ℃相比,23 ℃可促进拟南芥FCA的转录,使FCA蛋白水平升高,fca突变体对温度不敏感[10]。FCA通过诱导FT表达在高温下促进拟南芥开花[11]。与1年生拟南芥相比,一些多年生植物对温度变化的反应及其对开花的影响表现出多样性。例如,在多年生拟南芥的1个祖先近源种Boechera stricta中,与18 ℃相比,25 ℃处理下开花延迟[12];同样,在菊花Chrysanthemum morifolium中,也发现夏季温度升高能延迟菊花开花[13]。目前,对环境温度调控其开花的机理主要集中于模式植物中,木本植物种类繁多,且开花差异很大,关于木本植物中如何响应环境温度变化调控开花的机理仍不清楚。本研究通过对桂花Osmanthus fragrans OfFCA基因的同源克隆和定量聚合酶链式反应(PCR),分析OfFCA在不同温度下桂花不同花芽分化时期不同组织中的表达情况,初步探究OfFCA参与桂花花芽分化的调控作用,为桂花的花期调控、遗传改良以及新品种培育提供一定理论基础。
-
从浙江农林大学桂花种质资源圃中,选取株龄相同且生长一致的桂花‘堰虹桂’O. fragtans‘Yanhonggui’,分别于19和25 ℃处理。当处理0、10、20、30、40、50和60 d时,分别采集‘堰虹桂’的叶和花芽,一部分进行显微解剖结构观察;另一部分液氮处理后-80 ℃冻存,用于基因克隆和定量PCR分析。
-
石蜡切片制作参照刘涛等[14]的方法。主要步骤包括:①固定。采用体积比为18:1:1的700 mL·L-1乙醇、冰乙酸和甲醛将不同时期桂花的样品固定24 h以上。②切片。将经过脱水、透明、渗蜡、包埋等处理材料进行切片,厚度约12 μm。③染色及观察。固绿染色后用中性树脂封片,风干后,于显微镜(Axio Imager 2,日本)下观察。
-
取花芽分化时期约0.5 g的花芽或叶片,按照RNAprep pure Plant Kit试剂盒说明书提取RNA,RNase-free DNase Ⅰ(Takara)去除DNA。cDNA反转录参照Reverse Transcriptase M-MLV(Takara,大连)说明书,产物储存于-20 ℃备用。
-
通过前期转录组获得的FCA基因Unigene片段设计特异性引物(FCA-F:GCTATTCGTTGGAGGAGTT;FCA-R:GTTGTCTTGCGTAGTTGTC),以反转录的cDNA为模板进行PCR扩增,PCR反应体系如下:上下游定量引物(10 μmol·L-1)各1 μL,cDNA 1 μL,2×SYBR Premix Ex TaqⅡ(Tli RNaseH Plus)10 μL和双蒸水7 μL。PCR反应条件为:95 ℃预变性5 min;95 ℃变性30 s,60 ℃退火30 s,72 ℃延伸1 min;35次循环;72 ℃延伸10 min;4 ℃保存备用。PCR反应产物经10 mg·g-1琼脂糖凝胶电泳检测后回收,纯化,连接到pMD18-T载体,转化大肠埃希菌Escherichia coli DH5α感受态细胞,蓝白斑筛选阳性克隆,经PCR鉴定后送上海生工生物科技公司测序。
-
将克隆得到的OfFCA基因用DNAMAN软件进行多重比对序列,使用MEGA 7.0软件Neighbor-Joining法构建系统发育树;利用MultiLoc 2软件()进行亚细胞定位预测;在线工具TMHMM()和SignalP 5.0()分析OfFCA蛋白跨膜结构域、信号肽预测;利用ExPASy工具中的SOPMA软件预测蛋白质二级结构,用SWISS-MODEL(https://swissmodel.expasy.org/)对三级结构进行预测。
-
设计OfFCA荧光定量引物,送上海生工生物科技公司合成。荧光定量PCR反应体系为:SYBR Premix Ex TaqⅡ 10.0 μL,上下游定量引物(OfFCA-F:AGCATGTGTGTCCTGATGGA;OfFCA-R:GCTTATGATGCACCGGTTGT)各0.8 μL(10 μmol·L-1),cDNA 2.0 μL,双蒸水补齐至20.0 μL。反应程序如下:95 ℃预变性30 s,95 ℃ 5 s,60 ℃ 30 s,40个循环;95 ℃ 15 s,60 ℃ 1 min,95 ℃ 30 s,60 ℃ 15 s。3次生物学重复。采用2-△△CT法[15]计算OfFCA的相对表达量。
-
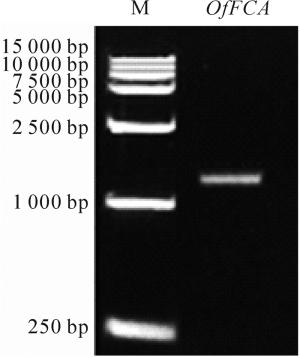
同源克隆获得桂花OfFCA基因cDNA序列,长度为1 319 bp(图 1),开放阅读框为864 bp,编码287个氨基酸,基因登录号为MK737873。通过DNAMAN软件比对发现:OfFCA与旋花科Convolvulaceae矮牵牛Ipomoea nil的IpFCA-like、茄科Solanaceae马铃薯Solanum tuberosum的SoFCA-like、胡麻科Pedaliaceae芝麻Sesamum indicum的SeFCA-like及玄参科Scrophulariaceae沟酸浆Erythranthe guttata的EryFCA和烟草Nicotiana attenuata的NiFCA-like较为相似,其氨基酸序相似度分别为76%、69%、68%、68%和68%(图 2)。
-
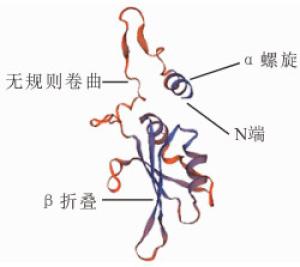
ExPASy软件结构域预测发现:OfFCA蛋白具有典型的RRM基序和WW结构域(图 2),其相对分子质量为70.6 kD,理论等电点值为5.12。OfFCA蛋白不存在信号肽,亚细胞定位预测显示:OfFCA蛋白定位于细胞质。蛋白结构三级如图 3所示。亲水性指数(GRAVY)为-0.565,表明OfFCA具有较好的亲水性。
-
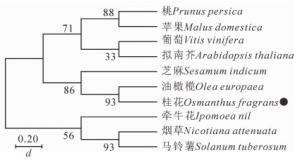
用MEGA7.0构建‘堰虹桂’与其他物种FCA之间的系统发育树(图 4)。结果显示:OfFCA与木犀科Oleaceae油橄榄Olea europaea的OlFCA和胡麻科芝麻的SeFCA具有较近的亲缘关系。
-
通过对不同温度下‘堰虹桂’不同花芽分化时期的石蜡切片发现:19 ℃处理约20 d后,‘堰虹桂’进入花序分化期,约30 d后进入小花分化期,40 d左右进入花萼和花瓣分化期,50 d后进入雄蕊分化期和雌蕊退化分化期。但在25 ℃处理下,‘堰虹桂’在30 d左右进入花序原基分化期且一直处于该时期(图 5),花芽分化进程显著延迟。以上现象说明低温19 ℃能够显著促进‘堰虹桂’的花芽分化进程,从而促进开花时间提前。

图 5 不同温度处理下‘堰虹桂’花芽分化进程
Figure 5. Flower bud differentiation period of Osmanthus fragrans'Y anhonggui' under different temperature treatments
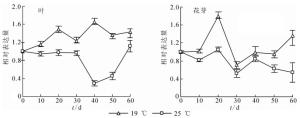
FCA基因是植物响应温度变化调控植物开花的重要基因,对桂花OfFCA基因进行定量表达检测发现:无论是在叶还是花芽组织中,OfFCA基因在19 ℃的表达均显著高于25 ℃(图 6),这说明OfFCA基因可响应相对低温19 ℃的变化,参与调控‘堰虹桂’的花芽分化。
-
环境温度不同于春化作用和冷胁迫,一般处于该物种生理学和非胁迫温度之间,广泛影响植物的生长发育。不同物种对环境温度的响应具有很大差异,例如,在低温条件下,拟南芥和水稻的开花时间均延迟[16-17],但是在朵丽蝶兰Doritaenopsis hybrid的研究中,低温能够诱导其成花转变,促进生理生化变化及花芽分化[18]。同时在烟草的研究中,低温也是促使烟草提前进入花期的重要因素之一[19]。本研究发现:与正常生长温度25 ℃相比,相对低温19 ℃显著促进‘堰虹桂’花芽分化进程,使开花时间提前(图 5)。目前,FCA同源基因在拟南芥等多个物种中已克隆得到,但是至今仍没有桂花OfFCA基因的相关报道。本研究从桂花秋桂品种‘堰虹桂’中分离得到1 319 bp的OfFCA基因(图 1),其开放阅读框为864 bp,编码287个氨基酸,基因登录号为MK737873。与其他物种FCA同源基因类似,OfFCA具有典型的RRM基序和WW结构域,与矮牵牛IpFCA-like的同源性最高(76%),且与其他物种包括马铃薯、芝麻、沟酸浆以及烟草的同源性均高达68%以上(图 2)。另外,OfFCA基因与木犀科油橄榄OeFCA和胡麻科芝麻SiFCA关系最近(图 4)。
环境温度对植物的花芽分化和开花具有重要的影响,其调控机制也存在很大的差异。FCA受转录和转录后调控,在拟南芥中,与16 ℃相比,23 ℃时FCA转录和蛋白质水平升高,进而促使成花转变使花期提前[20]。但在木本植物三叶橙Poncirus trifoliata中,与23 ℃相比,PtFCA1在较高的环境温度(27 ℃)显著下调,且35S::PtFCA1回补拟南芥fca-1突变体花期显著提前[21]。在桂花中发现,无论在叶和花芽中,19 ℃条件下OfFCA基因的表达均显著高于25 ℃(图 6)。据此可以推测,OfFCA响应环境温度变化参与桂花的花芽分化并使开花时间提前。在拟南芥等模式植物中,FCA通过抑制FLC以及SVP(SHORT VEGETATIVE PHASE)基因调控FT和SOC1的表达来促进花芽分化和开花时间[22],但是桂花OfFCA基因是否像模式植物一样,通过直接调控FLC和SVP基因的表达进而使FT和SOC1蛋白积累促进开花,还有待更进一步的验证。
目前,关于桂花的开花分子机制的报道比较少[23]。本研究通过对桂花的FCA基因克隆和表达分析,初步探究OfFCA影响环境温度变化调控桂花花芽分化的分子机制,对桂花的花期调控、遗传改良以及新品种培育提供一些理论基础。
Cloning and expression analysis of OfFCA gene at flower bud differentiation stages in Osmanthus fragrans
-
摘要:
目的 桂花Osmanthus fragrans是著名的香化植物,其花芽分化受到环境温度影响。研究环境温度对桂花花芽分化的影响对桂花的花期调控具有重要的指导意义。 方法 以桂花品种‘堰虹桂’O.fragrans ‘Yanhonggui’为材料,采用石蜡切片观察其花芽分化进程,运用聚合酶链式反应和实时荧光定量技术对影响温度FCA(FLOWERING LOCUS CA)基因分别进行克隆及表达特异性分析。 结果 克隆得到OfFCA cDNA序列长为1 319 bp,其开放阅读框为864 bp,编码287个氨基酸。序列比对及进化分析发现:OfFCA与木犀科Oleaceae油橄榄Olea europaea和胡麻科Pedaliaceae芝麻Sesamum indicum的FCA相似度较高,同源性可达68%以上。在桂花花芽分化的不同时期,无论叶还是花芽中,19℃环境低温下OfFCA基因的表达水平均显著高于25℃常温生长条件下的表达水平。 结论 桂花OfFCA基因响应环境相对低温的变化,参与桂花的花芽分化,使桂花的花期提前。 Abstract:Objective Sweet osmanthus (Osmanthus fragrans) is widely used in gardening as a fragrant plant. Its flower bud differentiation is significantly affected by ambient temperature. This research aims to find out the working mechanism of ambient temperature on the flower bud differentiation to help regulate flowering period of sweet osmanthus. Method Gene FCA (FLOWERING LOCUS CA) was studied using O. fragrans 'Yanhonggui' as the material, the process of flower bud differentiation was observed by paraffin section, and OfFCA was cloned and expression analysis was made by PCR and real-time PCR. Result The sequence length of OfFCA cDNA obtained by cloning was 1 319 bp, the Open Reading Frame(ORF) length was 864 bp, and 287 amino acids were encoded. Amino acid sequence alignment and evolutionary analysis showed that OfFCA was similar in FCA to Olea europaea, Oleaceae and Sesamum indicum, Pedaliaceae, with a homology of over 68%. The real time PCR demonstrated that the expression of OfFCA gene was higher at the low temperature (19℃) treatment than control temperature (25℃) in both leaves and flower buds at different flower bud differentiation stages. Conclusion Our work lay a foundation for the studying of regulating flowering time of O. fragrans by ambient temperature. -
Key words:
- forest tree breeding /
- Osmanthus fragrans /
- FCA gene /
- flower bud differentiation /
- gene expression
-
-
[1] 张艺能, 周玉萍, 陈琼华, 等.拟南芥开花时间调控的分子基础[J].植物学报, 2014, 49(4):469-482. ZHANG Yineng, ZHOU Yuping, CHEN Qionghua, et al. Molecular basis of flowering time regulation in Arabidopsis[J]. Chin Bull Bot, 2014, 49(4):469-482. [2] LIU Fuquan, MARQUARDT S, LISTER C, et al. Targeted 3' processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing[J]. Science, 2010, 327:94-97. [3] LIU Fuquan, QUESADA V, CREVILLÉN P, et al. The Arabidopsis RNA-binding protein FCA requires a lysine-specific demethylase 1 homolog to downregulate FLC[J]. Mol Cell, 2007, 28(3):398-407. [4] QUESADA V, MACKNIGHT R, DEAN C, et al. Autoregulation of FCA pre-mRNA processing controls Arabidopsis flowering time[J]. EMBO J, 2003, 22(12):3142-3152. [5] SUN Fan, LIU Chuanliang, ZHANG Chaojun, et al. A conserved RNA recognition motif (RRM) domain of Brassica napus FCA improves cotton fiber quality and yield by regulating cell size[J]. Mol Breed, 2012, 30(1):93-101. [6] LEE J H, CHO Y S, YOON H S, et al. Conservation and divergence of FCA function between Arabidopsis and rice[J]. Plant Mol Biol, 2005, 58(6):823-838. [7] MARQUARDT S, BOSS P K, HADFIELD J, et al. Additional targets of the Arabidopsis autonomous pathway members, FCA and FY[J]. J Exp Bot, 2006, 57(13):3379-3386. [8] 鲁旭, 孙芳, 华玉伟, 等.巴西橡胶树FCA基因的克隆及功能分析[J].广东农业科学, 2014, 41(15):116-120. LU Xu, SUN Fang, HUA Yuwei, et al. Cloning and functional analysis of FCA gene in Hevea brasiliensis[J]. Guangdong Agric Sci, 2014, 41(15):116-120. [9] MOON J, LEE H, KIM M, et al. Analysis of flowering pathway integrators in Arabidopsis[J]. Plant Cell Physiol, 2005, 46(2):292-299. [10] JUNG J H, SEO P J, AHN J H, et al. Arabidopsis RNA-binding protein FCA regulates microRNA172 processing in thermosensory flowering[J]. J Biol Chem, 2012, 287(19):16007-16016. [11] BLÁZQUEZ M A, AHN J H, WEIGEL D. A thermosensory pathway controlling flowering time in Arabidopsis thaliana[J]. Nat Genet, 2003, 33(2):168-171. [12] ANDERSON J T, LEE C R, MITCHELL-OLDS T. Life-history QTLs and natural selection on flowering time in Boechera stricta, a perennial relative of Arabidopsis[J]. Evolution, 2011, 65(3):771-787. [13] NAKANO Y, HIGUCHI Y, SUMITOMO K, et al. Flowering retardation by high temperature in chrysanthemums:involvement of FLOWERING LOCUS T-like 3 gene repression[J]. J Exp Bot, 2013, 64(4):909-920. [14] 刘涛, 任莉萍, 曹沛沛, 等.菊花不同时期各组织器官石蜡切片制作条件的优化[J].南京农业大学学报, 2016, 39(5):739-746. LIU Tao, REN Liping, CAO Peipei, et al. The paraffin section making conditions of chrysanthemum different tissues in different period[J]. J Nanjing Agric Univ, 2016, 39(5):739-746. [15] LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J]. Methods, 2001, 25(4):402-408. [16] WIGGE P A. Ambient temperature signalling in plants[J]. Curr Opin Plant Biol, 2013, 16(5):661-666. [17] 宋远丽, 栾维江.水稻开花的光温调控分子机理[J].中国水稻科学, 2012, 26(4):383-392. SONG Yuanli, LUAN Weijiang. Regulatory pathways of rice flowering in different light and temperature conditions[J]. Chin J Rice Sci, 2012, 26(4):383-392. [18] 张迟, 周庐萍, 罗小燕, 等.低温对朵丽蝶兰成花过程中碳水化合物及糖转运蛋白基因表达的影响[J].中国农业科学, 2011, 44(8):1670-1677. ZHANG Chi, ZHOU Luping, LUO Xiaoyan, et al. Relative cold-induced flowering arouse fluctuation on carbohydrates and expression of genes related to sugar transport in Doritaenopsis hybrid[J]. Sci Agric Sin, 2011, 44(8):1670-1677. [19] 李元元.低温诱导烟草早花研究与烟草MADS-box基因的同源克隆[D].北京: 中国农业科学院, 2011. LI Yuanyuan. Research on Cold Induced Early Flowering and Homologous Cloning of MADS-box Genes in Tobacco[D]. Beijing: Chinese Academy of Agricultural Sciences, 2011. [20] CHO H J, KIM J J, LEE J H, et al. SHORT VEGETATIVE PHASE (SVP) protein negatively regulates miR172 transcription via direct binding to the pri-miR172a promoter in Arabidopsis[J]. FEBS Lett, 2012, 586(16):2332-2337. [21] AI Xiaoyan, ZHANG Jinzhi Z, LIU Tianjia, et al. PtFCA from precocious trifoliate orange is regulated by alternative splicing and affects flowering time and root development in transgenic Arabidopsis[J]. Tree Genet Genomes, 2016, 12(5):85. [22] CAPOVILLA G, SCHMID M, POSÉD. Control of flowering by ambient temperature[J]. J Exp Bot, 2014, 66(1):59-69. [23] 蒋琦妮, 付建新, 张超, 等.桂花OfAP1基因的克隆及表达分析[J].浙江农林大学学报, 2019, 36(4):664-669. JIANG Qini, FU Jianxin, ZHANG Chao, et al. cDNA cloning and expression analysis of OfAP1 in Osmanthus fragrans[J]. J Zhejiang A&F Univ, 2019, 36(4):664-669. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2020.02.001






 下载:
下载: