-
土壤有机质是土壤的重要组成部分,是土壤团聚体形成的胶结物质,同时也是衡量土壤肥力的重要指标,其含量和质量一定程度上影响着土壤团聚体的形成和稳定[1]。土壤酶作为直接参与土壤生态系统碳、氮、磷代谢的重要动力,主要由高等植物根系和土壤动物分泌、土壤微生物生命活动释放的一类具有高度催化作用的蛋白质组成[2]。土壤酶中的水解酶具有高效催化水解作用,参与土壤中有机物的转化,能裂解有机化合物中的糖苷键、脂键和肽键等,把高分子化合物水解成植物和微生物可利用的营养物质,在促进土壤有机物质分解和腐殖质合成、生态系统的物质循环及能量流动中起着重要作用[3-4]。土壤酶活性作为土壤质量的生物活性指标,比土壤物理、化学指标对土壤环境的变化更敏感[5],它可以从本质上反映土壤碳、氮、磷的转化强度,对土壤生态系统中各种生物化学过程调控的功能发挥具有重要的意义[6]。团聚体作为土壤结构的基本单元,能协调土壤中的水、肥、气、热以及维持和稳定不同土壤层,是形成良好土壤结构的物质基础[7]。不同粒径团聚体不仅决定着土壤形态特征、孔隙的分布和数量等土壤理化性质,更在土壤养分供应、保持及转化等方面发挥着重要作用[7-8]。岩溶石漠化是中国西南地区主要的土地退化形式,虽然石漠化整体扩展趋势得到了有效遏制,生态状况稳定好转[9],但局部石漠化土地仍在扩展,加之岩溶生态系统属于脆弱生态区,石漠化治理具有长期性和艰巨性特点[10]。多年来,石漠化区域进行了大规模的退耕还林还草等植被恢复活动,很多研究关注植被恢复过程对土壤肥力的影响,主要集中在土壤养分空间分布及在石漠化恢复过程中的变化规律[11]、土壤水文结构特征[12]、不同植被恢复模式的优劣[13]、土壤微生物群落结构特征[14]、人为活动等对土壤物理化学性质的影响等方面,关于土壤酶活性,尤其是水解酶的分布特征鲜有报道。此外,以往研究土壤酶活性时多以过筛后均匀混合的土壤样品作为研究对象[6, 15-18],很少考虑到团聚体中酶活性的变化情况。事实上,不同粒径团聚体的形成环境和胶结类型不同,常导致团聚体的稳定性及内部物质组成等表现出特殊性,进而导致不同粒径团聚体之间土壤酶活性[5]和养分的差异[15],因此,探究岩溶区土壤团聚体碳、氮、磷含量及水解酶活性特征具有重要意义。为此,本研究选择滇中高原典型岩溶石漠化区为研究区域,以4种石漠化程度(潜在、轻度、中度、重度)的土壤为研究对象,探究土壤团聚体分布特征以及碳、氮、磷和水解酶活性在团聚体中的分布情况,为岩溶石漠化区土壤改良和植被恢复提供科学依据。
HTML
-
研究区位于云南省昆明市石林县(24°38′~24°58′N,103°11′~103°29′E),海拔1 500~1 900 m,属亚热带低纬度高原山地季风气候,干湿季差异明显,年均气温约16.0 ℃,年均降水量946.0 mm,多集中在5—10月。该县石漠化土地约占国土面积的20%,以中度为主,其次为轻度、强度石漠化土地。境内为滇中岩溶高原红壤分布地带,土壤pH为4.5~6.5,主要土壤类型为红壤、紫色土和水稻土,以石灰岩红壤分布最广,约85.2%,石灰岩红壤普遍质地黏重,通透性差,有机质含量低。2016年石林县林业局调查结果显示:石林岩溶石漠生境土地面积约2.9 万hm2,包括轻度1.1 万hm2、中度1.3 万hm2和重度0.5 万hm2[19]。研究区植物类型大都以由先锋物种构成的低矮灌丛为主,多生长于裸露岩石的缝隙中,无明显的乔木层,成零星状分布,灌木与同一地区的亚热带常绿阔叶林的次生林灌木相近,植被覆盖率均较低。优势植被主要有云南松Pinus yunnanensis、桤木Alnus cremastogyne、柏木Cupressus funebris、清香木Pistacia weinmannifolia、云南含笑Michelia yunnanensis、茅叶荩草Arthraxon prionodes、沙针Osyris wightiana、地石榴Ficus tikoua、薄皮木Leptodermis oblonga、四脉金茅Eulalia quadrinervis、铁仔Myrsine africana、破坏草Ageratina adenophora等。
-
2018年4月,选择发育类型基本相同,海拔、坡度、坡向等相近,成土母质相同且具有代表性的不同石漠化程度(潜在、轻度、中度和重度)土壤为研究对象。石漠化程度的划分参照王宇等[20]的方法。随机设置面积为10 m×10 m的3个样方,每个样方内按“S”型设置5个采样点。由于石漠化区基岩裸露、土层浅薄,采集0~10 cm土层土壤样品。采样时先去除凋落物及腐殖质层,再采集土样,装入自封袋,带回实验室进行后续分析处理。土样沿自然结构掰成直径约1 cm的土块,去除动植物残体后置于室内阴凉通风处风干,风干后取一部分土样用于测定全土理化性质及酶活性,剩余部分用干筛法[21]分离出粒径<0.25 mm、粒径0.25~2.00 mm、粒径>2.00 mm团聚体用于测定土壤团聚体理化性质及酶活性。土壤团聚体的分离方法如下:将土样放在筛上,按筛孔大小(直径0.25 mm、2.00 mm)套好,用手摇动筛子,直至筛上的土团不再下漏为止,从而将土样分成粒径>2.00 mm、粒径0.25~2.00 mm、粒径<0.25 mm共3个粒级,最后收集筛上的土样,分别称量,计算各粒径级土壤团聚体质量分数。样地基本概况如表1所示。
石漠化
程度海拔/m 经度(E) 纬度(N) pH 有机碳/
(g·kg−1)全氮/
(g·kg−1)全磷/
(g·kg−1)主要植被 裸岩面
积比例/
%潜在 1 853~1 855 103°20′41.8″~
103°20′43.7″24°46′04.1″~
24°46′05.8″4.68 24.20±3.14 b 1.57±0.27 c 1.08±0.15 c 云南松、云南含笑、清
香木、铁仔、四脉金
茅、茅叶荩草、破坏草0~30 轻度 1 852~1 862 103°20′37.6″~
103°20′39.5″24°46′03.4″~
24°46′06.0″5.35 32.60± 2.38 a 2.18±0.20 b 0.94±0.15 c 云南松、云南杨梅、铁
仔、沙针、茅叶荩草、
破坏草、鬼针草30~50 中度 1 831~1 833 103°20′40.9″~
103°20′48.5″24°46′25.7″~
24°46′30.6″4.87 13.74±1.64 c 1.05±0.09 d 1.69±0.31 b 云南松、小雀花、薄皮
木、铁仔、破坏草50~70 重度 1 822~1 833 103°20′28.3″~
103°20′31.5″24°46′12.5″~
24°46′13.6″5.66 34.34±5.49 a 2.73±0.43 a 2.74±0.74 a 云南松、铁仔、茅叶荩
草、破坏草、鬼针草>70 说明:有机碳、全氮、全磷数据为平均值±标准差;同列不同小写字母表示不同石漠化程度间土壤养分差异显著(P<0.05)。云南杨 梅Myrica nana;鬼针草Bidens pilosa;小雀花Campylotropis polyantha Table 1. Basic information of the plot
-
土壤有机碳、全氮、全磷、pH的测定参照鲍士旦[22]的方法进行;淀粉酶采用3,5-二硝基水杨酸比色法[23]测定,脲酶采用靛酚蓝比色法[24]测定,土壤β-葡萄糖苷酶和酸性磷酸酶采用硝基苯底物比色法[23]测定,并测定了不同粒径团聚体及混合土壤的各项指标。
土壤团聚体平均质量直径(DMW,mm)的计算公式[25]:
式(1)中:di为第i粒径团聚体直径的平均值(mm);wi为第i粒径团聚体的质量分数(%)。团聚体贡献率计算公式[26]:
土壤酶活性几何平均数(M)[27]是综合评价土壤酶活性的指标,也是指示土壤生物质量的综合指标,可以反映土壤质量的变化。计算公式为:
式(3)中:EAmy为淀粉酶活性(μg·g−1·h−1);EUre为脲酶活性(μg·g−1·h−1);EGlu为β-葡萄糖苷酶活性(μg·g−1·h−1);EACP为酸性磷酸酶活性(μg·g−1·h−1)。
-
运用Excel 2010、SPSS 21.0对数据进行分析处理。采用单因素方差分析(one-way ANOVA)检验相同粒径不同石漠化程度之间的差异,同时检验相同石漠化程度不同粒径之间的差异;采用双因素方差分析(two-way ANOVA)检验粒径、石漠化程度及其交互因子对土壤团聚体碳、氮、磷及其土壤酶活性的影响。显著性水平为0.05。
1.1. 研究区概况
1.2. 样地布设与样品采集
1.3. 样品的测定与分析
1.4. 数据处理与分析
-
同种石漠化程度不同粒径土壤团聚体组成比例随粒径的增大而增大。不同石漠化程度土壤团聚体质量分数从大到小依次为粒径>2.00 mm、粒径0.25~2.00 mm、粒径<0.25 mm,粒径>2.00 mm和粒径0.25~2.00 mm的质量分数分别是粒径<0.25 mm的4.26和3.03倍。不同石漠化程度土壤团聚体组成均以粒径>2.00 mm为主,其中重度石漠化土壤团聚体质量分数高达58.47%,显著高于中度石漠化土壤(P<0.05);重度石漠化土壤粒径0.25~2.00 mm团聚体质量分数显著低于其他石漠化程度(P<0.05);不同石漠化程度土壤团聚体平均质量直径(DMW)从大到小依次为重度、潜在、轻度、中度,且潜在和轻度石漠化土壤的DMW差异不显著(P>0.05)(表2)。
石漠化程度 土壤团聚体质量分数/% DMW/mm 粒径<0.25 mm 粒径0.25~2.00 mm 粒径>2.00 mm 潜在 12.00±0.04 aC 35.60±0.06 abB 52.20±0.09 abA 2.25±0.26 ab 轻度 11.60±0.04 aC 39.20±0.08 aB 49.07±0.11 abA 2.17±0.31 ab 中度 14.50±0.07 aB 39.86±0.08 aA 45.50±0.10 bA 2.05±0.28 b 重度 10.07±0.04 aC 31.47±0.08 bB 58.47±0.11 aA 2.41±0.31 a 说明:数据为平均值±标准差;同列不同小写字母表示相同粒径不同石漠化程度土壤团聚体质量分数差异显著(P<0.05);同行不同 大写字母表示相同石漠化程度不同粒径团聚体质量分数差异显著(P<0.05) Table 2. Composition characteristics of soil aggregates at different levels of rocky desertification
-
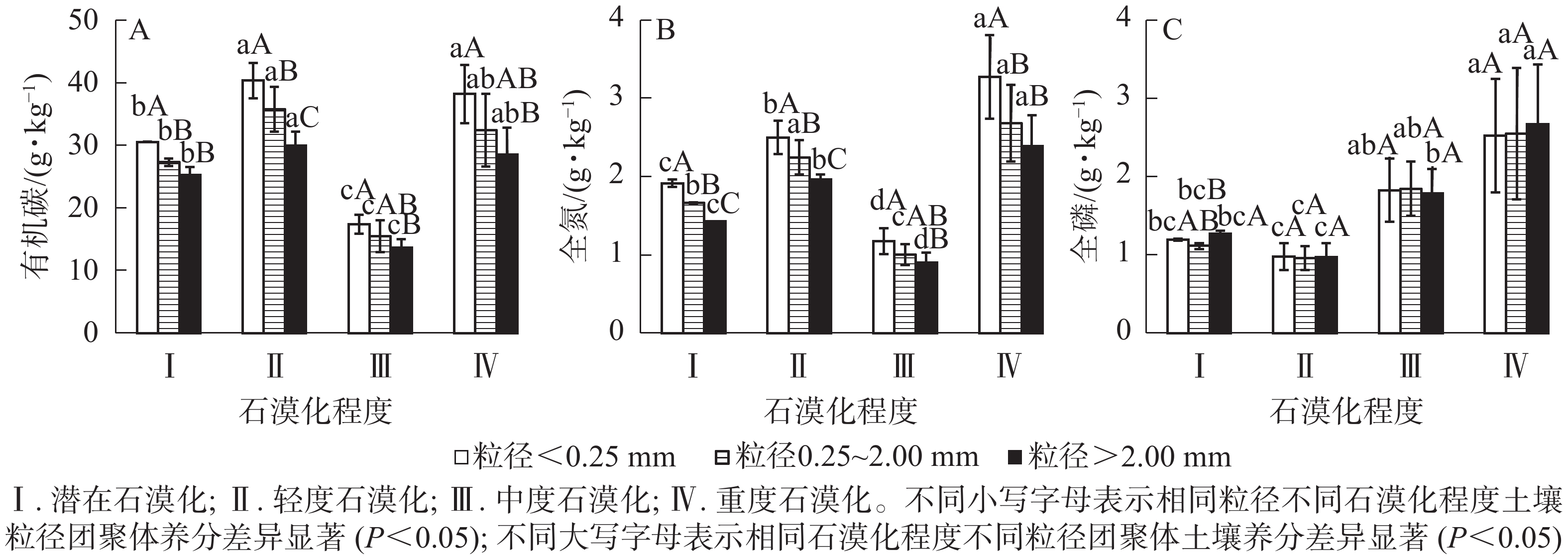
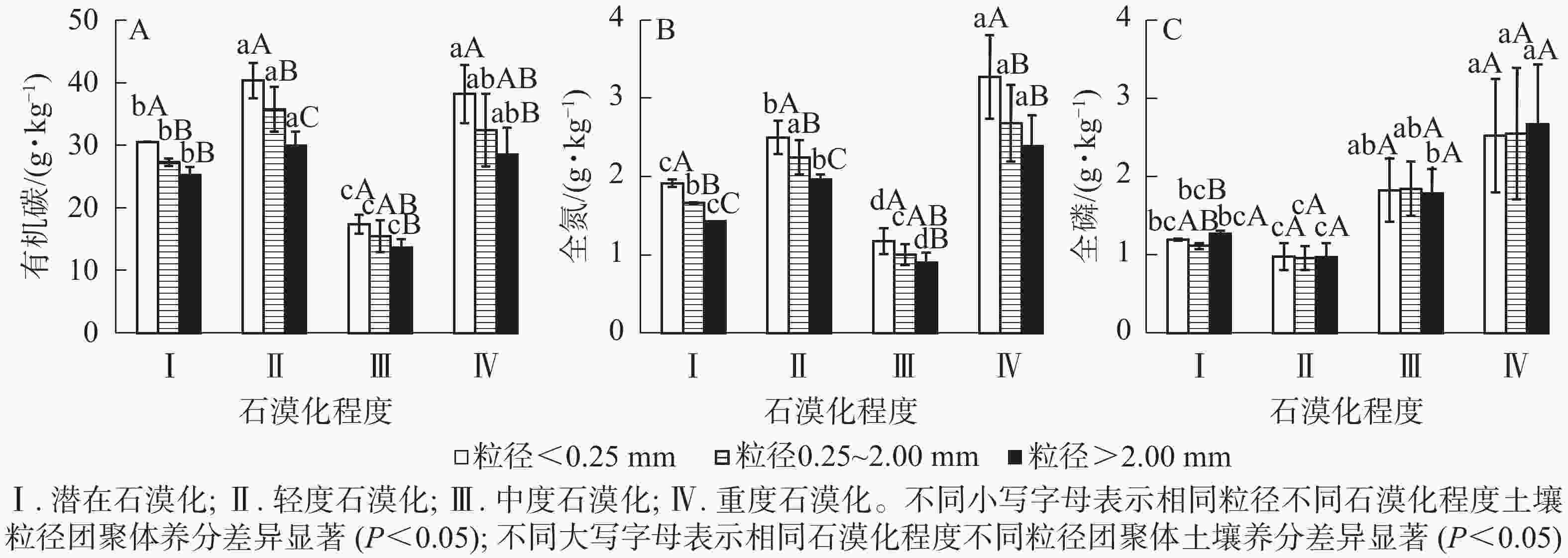
土壤有机碳质量分数在粒径<0.25 mm的团聚体中,为17.40~40.34 g·kg−1,在粒径0.25~2.00 mm的团聚体中,为15.45~35.75 g·kg−1,在粒径>2.00 mm的团聚体中,为13.65~29.94 g·kg−1,各粒径土壤团聚体有机碳质量分数随粒径的增大而降低,且在小粒径团聚体中有机碳质量分数较高。相同粒径不同石漠化程度土壤中,潜在和中度石漠化土壤有机碳质量分数显著低于轻度、重度石漠化土壤(图1A)(P<0.05)。表3表明:粒径和石漠化程度对土壤有机碳影响显著(P<0.05),但粒径和石漠化程度的交互作用对土壤有机碳影响不显著(P>0.05)。
变异来源 P 有机碳 全氮 全磷 淀粉酶 脲酶 β-葡萄糖苷酶 酸性磷酸酶 粒径 0.000* 0.000* 0.939 0.000* 0.000* 0.000* 0.002* 石漠化程度 0.000* 0.000* 0.000* 0.000* 0.000* 0.000* 0.000* 粒径×石漠化程度 0.266 0.322 0.999 0.000* 0.298 0.098 0.737 说明:*表示差异显著(P<0.05) Table 3. Two-factor variance analysis of soil organic carbon, total nitrogen, total phosphorus and soil enzyme activity
土壤全氮质量分数在粒径<0.25 mm时,为1.17~3.27 g·kg−1,在粒径0.25~2.00 mm时,为1.00~2.68 g·kg−1,在粒径>2.00 mm时,为0.90~2.39 g·kg−1,各粒径土壤团聚体全氮质量分数随粒径的增大而降低(图1B)。相同粒径团聚体不同石漠化程度土壤中,潜在和中度石漠化土壤全氮质量分数显著低于重度、轻度石漠化土壤(P<0.05),其中粒径<0.25 mm和>2.00 mm的石漠化土壤全氮质量分数从大到小依次为重度、轻度、潜在、中度。表3表明:粒径和石漠化程度对土壤全氮影响显著(P<0.05),但粒径和石漠化程度的交互作用对全氮影响不显著(P>0.05)。
土壤全磷质量分数在粒径<0.25 mm时,为0.98~2.52 g·kg−1,在粒径0.25~2.00 mm时,为0.96~2.55 g·kg−1,在粒径>2.00 mm时,为0.97~2.67 g·kg−1,各粒径团聚体全磷质量分数差异不显著(图1C)(P>0.05)。表3表明:石漠化程度对土壤全磷影响显著(P<0.05),但粒径、粒径和石漠化程度的交互作用对全磷影响不显著(P>0.05)。
-
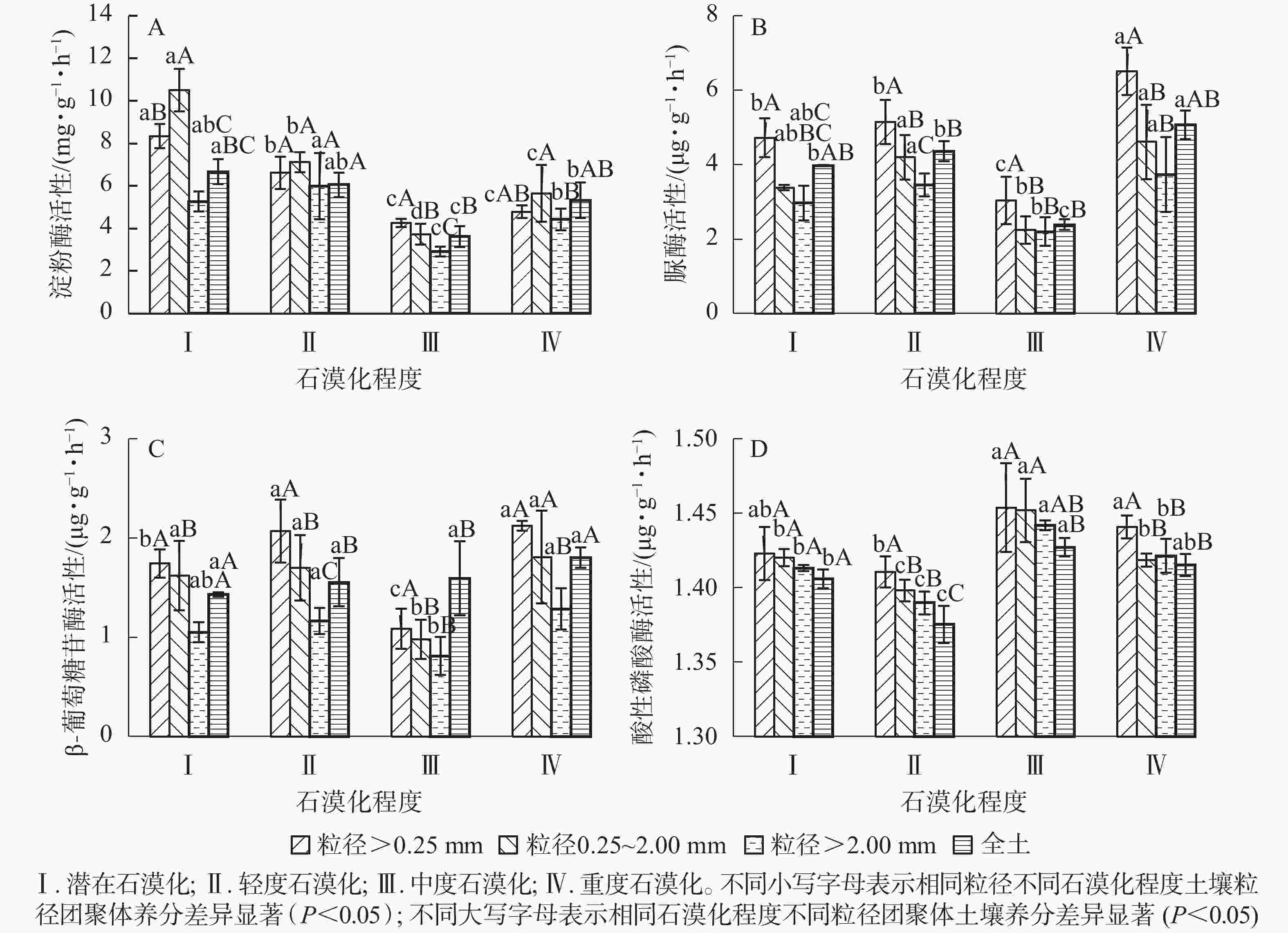
不同粒径土壤团聚体淀粉酶活性为2.91~10.51 mg·g−1·h−1,各石漠化程度土壤酶活性从大到小依次为潜在、轻度、重度、中度(图2A)。粒径<0.25 mm及粒径0.25~2.00 mm的团聚体土壤酶活性较高,均值分别为5.99、6.75 mg·g−1·h−1;粒径>2.00 mm的团聚体土壤酶活性最低(4.64 mg·g−1·h−1)。粒径<0.25 mm的团聚体中,潜在、轻度与中度、重度石漠化程度土壤淀粉酶活性差异显著(P<0.05);粒径0.25~2.00 mm的团聚体中,各石漠化程度土壤酶活性差异显著(P<0.05);粒径>2.00 mm的团聚体中,中度石漠化土壤淀粉酶活性显著低于潜在、轻度、重度石漠化土壤。潜在、中度石漠化不同粒径土壤酶活性差异显著,轻度、重度石漠化程度各粒径土壤酶活性差异不显著。双因素方差分析表明:粒径、石漠化程度及粒径和石漠化程度的交互作用均对淀粉酶活性有显著影响(P<0.05)(表3)。
-
不同粒径团聚体土壤脲酶活性为2.20~6.51 μg·g−1·h−1,各石漠化程度酶活性从大到小依次为重度、轻度、潜在、中度。由图2B可知:不同粒径团聚体土壤脲酶活性随粒径增大而降低。由差异性分析可知:粒径<0.25 mm的团聚体中,潜在、轻度石漠化与中度、重度石漠化土壤酶活性差异显著(P<0.05),各石漠化程度粒径<0.25 mm的团聚体土壤酶活性显著高于粒径0.25~2.00 mm、粒径>2.00 mm的团聚体酶活性。由表3可知:粒径、石漠化程度对土壤脲酶活性影响显著(P<0.05)。
-
不同粒径团聚体土壤β-葡萄糖苷酶活性为0.81~2.12 μg·g−1·h−1,各石漠化程度土壤酶活性从大到小依次为重度、轻度、潜在、中度。由图2C可知:不同粒径团聚体酶活性随粒径增大而降低。粒径<0.25 mm和粒径0.25~2.00 mm的团聚体中,中度石漠化土壤酶活性显著低于潜在、轻度、重度石漠化土壤酶活性(P<0.05)。由表3可知:粒径、石漠化程度对土壤β-葡萄糖苷酶活性影响显著(P<0.05),粒径和石漠化程度的交互作用对β-葡萄糖苷酶活性影响不显著(P>0.05)。
-
不同粒径团聚体土壤酸性磷酸酶活性为1.39~1.45 μg·g−1·h−1,均值为1.42 μg·g−1·h−1 (图2D)。不同石漠化程度及不同粒径团聚体土壤酸性磷酸酶活性差异均较小。粒径和石漠化程度均对酸性磷酸酶活性影响显著(P<0.05),但粒径和石漠化程度的交互作用对其无显著影响(P>0.05)(表3)。
-
从表4可知:潜在、轻度、中度和重度石漠化土壤团聚体酶活性几何平均数分别为:12.33~17.67、13.40~17.68、9.25~11.91、13.68~18.17 μg·g−1·h−1。中度石漠化土壤团聚体各粒径酶活性几何平均数均显著低于潜在、轻度、重度石漠化(P<0.05),而潜在、轻度、重度石漠化土壤各粒径团聚体酶活性几何平均数差异不显著(P>0.05)。从土壤团聚体来看,土壤酶活性几何平均数随团聚体粒径的增大而减小,说明小团聚体更利于土壤酶活性的积累。
石漠化程度 几何平均数/(μg·g−1·h−1) 全土 粒径<0.25 mm 粒径0.25~2.00 mm 粒径>2.00 mm 潜在 17.67±1.09 aA 16.86±0.58 aAB 12.33±1.06 aC 15.18±0.04 aB 轻度 17.68±0.73 aA 16.27±1.35 aB 13.40±0.81 aC 15.37±0.78 aB 中度 11.91±1.21 bA 10.40±1.12 bB 9.25±0.95 bB 11.78±1.05 bA 重度 18.17±1.23 aA 16.75±3.39 aA 13.68±1.96 aB 16.53±1.21 aA 说明:数值为平均值±标准差;同列不同小写字母表示相同粒径不同石漠化程度土壤团聚体酶几何平均数差异显著(P<0.05);同行 不同大写字母表示相同石漠化程度不同粒径团聚体土壤酶活性几何平均数差异显著(P<0.05) Table 4. Ceometric averages of soil enzymes activities
-
不同石漠化程度下,土壤淀粉酶、脲酶、β-葡萄糖苷酶活性及土壤有机碳、全氮、全磷贡献率均为粒径0.25~2.00 mm、粒径>2.00 mm的团聚体显著高于粒径<0.25 mm(表5,表6)(P<0.05)。酸性磷酸酶活性在粒径>2.00 mm的团聚体中贡献率显著高于粒径<0.25 mm、粒径0.25~2.00 mm的团聚体;全氮和全磷在相同石漠化程度不同粒径团聚体中各粒级贡献率差异显著(P<0.05)。重度石漠化土壤淀粉酶、脲酶、β-葡萄糖苷酶活性、有机碳、全氮、全磷各粒级贡献率差异显著(P<0.05)。本研究表明:粒径>2.00 mm的团聚体在土壤养分含量中不是最高,但在各石漠化程度中都占了优势,因此它的贡献率最高。
土壤养分 石漠化程度 养分贡献率/% 粒径<0.25 mm 粒径0.25~2.00 mm 粒径>2.00 mm 有机碳 潜在 15.80±0.01 aC 39.69±0.02 aB 55.49±0.01 aA 轻度 14.52±0.02 abB 43.02±0.04 aA 49.38±0.06 aA 中度 18.54±0.03 aB 45.03±0.05 aA 45.21±0.05 aA 重度 11.15±0.03 bC 29.64±0.06 bB 48.82±0.09 aA 全氮 潜在 15.27±0.01 abC 37.30±0.02 abB 48.48±0.00 aA 轻度 13.47±0.02 abC 40.41±0.02 aB 44.68±0.05 abA 中度 16.12±0.02 aB 38.09±0.04 abA 38.70±0.02 bA 重度 12.02±0.03 bC 30.91±0.07 bB 51.05±0.089 aA 全磷 潜在 13.45±0.01 abC 35.18±0.00 bcB 60.79±0.01 aA 轻度 12.11±0.01 bcC 40.01±0.04 abB 50.67±0.06 bA 中度 15.63±0.02 aC 43.79±0.03 aB 47.97±0.02 bA 重度 9.25±0.03 cC 29.02±0.07 cB 56.68±0.09 abA 说明:数值为平均值±标准差;同列不同小写字母表示相同粒径不同石漠化程度土壤团聚体养分贡献率差异显著(P<0.05);同行不 同大写字母表示相同石漠化程度不同粒径团聚体土壤养分贡献率差异显著(P<0.05) Table 5. Nutrient contribution rates of soil aggregates at different rocky desertification degrees
土壤酶 石漠化程度 酶活性贡献率/% 粒径<0.25 mm 粒径0.25~2.00 mm 粒径>2.00 mm 淀粉酶 潜在 15.53±0.01 aC 55.00±0.03 aA 41.62±0.00 aB 轻度 12.77±0.01 bB 46.53±0.07 abA 48.22±0.11 aA 中度 17.13±0.01 aB 41.48±0.05 bcA 36.91±0.05 aA 重度 9.02±0.02 cC 33.58±0.10 cB 48.95±0.09 aA 脲酶 潜在 14.68±0.01 aB 29.66±0.01 aA 39.36±0.06 aA 轻度 13.74±0.01 aB 37.73±0.05 aA 38.82±0.03 aA 中度 18.20±0.02 aB 37.41±0.03 aA 41.59±0.06 aA 重度 13.78±0.05 aC 32.90±0.14 aB 45.02±0.07 aA β-葡萄糖苷酶 潜在 15.08±0.00 aB 39.44±0.07 abA 38.73±0.03 aA 轻度 16.04±0.04 aB 44.98±0.17 aA 37.31±0.06 aA 中度 10.04±0.02 bB 25.01±0.04 bA 23.13±0.02 bA 重度 11.82±0.03 abB 32.19±0.12 abA 41.23±0.06 aA 酸性磷酸酶 潜在 12.56±0.01 abB 12.52±0.01 abB 52.99±0.01 abA 轻度 12.00±0.01 abB 11.89±0.01 abB 49.59±0.04 bA 中度 14.86±0.02 aB 14.82±0.02 aB 45.73±0.02 bA 重度 10.22±0.03 bB 10.06±0.03 bB 58.75±0.09 aA 说明:数值为平均值±标准差;同列不同小写字母表示相同粒径不同石漠化程度土壤团聚体酶活性贡献率差异显著(P<0.05);同行 不同大写字母表示相同石漠化程度不同粒径团聚体土壤酶活性贡献率差异显著(P<0.05) Table 6. Contribution rates of soil aggregates enzyme activities in different degrees of rocky desertification
2.1. 不同石漠化程度土壤团聚体组成及稳定性
2.2. 不同石漠化程度土壤团聚体碳、氮、磷特征
2.3. 不同石漠化程度土壤团聚体酶活性特征
2.3.1. 淀粉酶活性
2.3.2. 脲酶活性
2.3.3. β-葡萄糖苷酶活性
2.3.4. 酸性磷酸酶活性
2.4. 不同石漠化程度土壤团聚体养分与酶活性综合评价
2.4.1. 土壤团聚体酶活性几何平均数
2.4.2. 各粒径土壤团聚体碳、氮、磷与酶活性的贡献率
-
土壤团聚体的组成比例及稳定性是评价土壤结构的重要指标,稳定的团聚体可以减少土壤可侵蚀性及提升土壤肥力[28]。土壤颗粒在持久性胶结物质的作用下形成粒径<0.25 mm的微团聚体,具有抵抗机械破坏和调节土壤水、肥、气、热以及各种生物活动等功能[29],之后在瞬时性及暂时性的胶结物质作用下逐级形成粒径>0.25 mm的大团聚体[30]。团聚体的比例能反映不同石漠化程度土壤结构的稳定性。研究区不同石漠化程度土壤粒径团聚体组成比例随粒径的增大而增大,且粒径>0.25 mm团聚体质量分数均值高达87.84%,说明该研究区土壤团聚性较强,分散性较弱,这与黄永珍等[31]、廖洪凯等[32]的研究结果基本一致。该研究认为:表征土壤团聚体结构稳定性的土壤团聚体平均质量直径从大到小依次为重度、潜在、轻度、中度,重度石漠化土壤团聚体平均质量直径较高,可能是由于该区虽然岩石裸露率高,植被覆盖率低,但岩石空隙草本植物较多。有研究认为:天然草地土壤团聚体稳定性高于灌木林地[33],潜在石漠化和轻度石漠化由于植被覆盖率及种类较多,导致植被凋落物量大且凋落物分解速率显著高于其他石漠化程度植被凋落物,使得土壤活性有机质的积累促进了较大粒径团聚体的形成[34]。中度石漠化由于植被覆盖率较低,受到的人为干扰更多,增强了对表层土壤大团聚体的破坏。
-
土壤团聚体粒径对酶活性的分布有重要影响,研究区土壤团聚体酶几何平均数、脲酶、β-葡萄糖苷酶、酸性磷酸酶活性均随粒径的增大而减小,淀粉酶活性在粒径<0.25 mm和粒径0.25~2.00 mm的团聚体中较高,在粒径>2.00 mm的团聚体中最低,这与姬秀云等[35]的研究结果一致。朱家琪等[36]研究也显示:蔗糖酶、脲酶和酸性磷酸酶活性随着团聚体粒径的减小而增大,其中粒径0.25 mm团聚体的酶活性最高。NIE等[37]研究表明:大团聚体不利于土壤酶活性的累积。此外,在植被恢复过程中,小粒径团聚体先形成[17],因此导致包裹在大团聚体内部的小粒径团聚体具有稳定的有机物质、酶及其底物。
本研究显示:不同石漠化程度土壤团聚体有机碳及全氮质量分数均为重度和轻度石漠化显著高于潜在和中度石漠化,粒径<0.25 mm团聚体的有机碳、全氮质量分数均显著高于粒径0.25~2.00 mm和粒径>2.00 mm的团聚体。这是因为土壤团聚体对养分的吸附能力与比表面积呈正比,在质量相同的情况下,粒径越小的土壤团聚体,比表面积越大[31],其次,有机质在团聚体形成的最开始阶段起到的作用更突出,所以较小粒级团聚体中的有机碳高于较大的粒径团聚体[38]。马瑞萍等[39]研究显示:粒径<0.25 mm的团聚体总有机碳含量较高,可能是由于微团聚体中有机碳主要以稳定的腐殖质碳为主,而大团聚体中有机碳则主要以易分解、矿化的活性有机碳为主。因此,小团聚体中有机碳不断积累,而大团聚体中由于活性有机碳的分解、矿化,导致总有机碳含量不断降低。粒径<0.25 mm的团聚体全氮高,主要受到有机碳含量以及小粒级团聚体对铵根离子(NH4 +)吸附力较高的影响[40]。不同石漠化程度土壤全磷含量在各粒级团聚体中分布较均匀,这与卢怡等[26]的研究结果一致。可能是因为全磷含量在土壤中不易迁移,从而使它在土壤中比较稳定。
本研究区重度石漠化土壤团聚体养分、酶活性均较高,这与大多数的研究结果有所不同,可能是由于该区虽然岩石裸露率高,但雨水冲击等作用导致局部土壤肥沃,岩石空隙间生长了大量的草本植物,其发达的根系,使石漠化程度严重,石灰岩的溶解程度加大,分解出的有机质增多[41]。另外,有研究显示:岩石裸露率较高的地方养分元素并非一定减少,而是呈一定的正相关性,因为基岩裸露的过程其实也是石面土壤被侵蚀、养分物质在岩隙和石槽中沉积的过程[42],这与杨丹等[17]、张伟等[42]的研究结果一致。中度石漠化区土壤团聚体有机碳、全氮含量显著低于潜在、轻度、重度石漠化,淀粉酶、脲酶、β-葡萄糖苷酶活性均最低,酸性磷酸酶活性最高。卢怡等[26]研究表明:土壤养分与酶活性之间有显著相关性。吴丽芳等[43]研究显示:不同石漠化程度土壤有机碳、全氮与淀粉酶、脲酶、β-葡萄糖苷酶活性呈显著正相关,与酸性磷酸酶活性呈负相关,表明酶活性对土壤中各形态有机碳及全氮之间的相互转化起到重要作用。还有研究表明:有机质含量高的土壤团聚体中酶活性较高[44]。研究区中度石漠化土壤酶活性较低,是由于该区属于植被恢复初期,植被覆盖率较低,凋落物量较少,因此土壤养分含量较低。而潜在石漠化与轻度石漠化区植被覆盖率相对较高,有一定的凋落物及腐殖质覆盖在土壤表层,导致底物增加,进而诱导酶活性增强[19]。酸性磷酸酶活性在不同石漠化程度土壤团聚体中差异不明显,是因为酸性磷酸酶活性是影响土壤磷素有效性的重要因子,而土壤中的磷素大部分是以迟效性状态存在[45],在土壤中移动性较小。土壤碳、氮已被广泛证实是影响土壤酶活性的主要因素[17]。因此要提高岩溶石漠化区土壤酶活性,应优先提高土壤有机质含量,而进行植被修复又是提高岩溶区土壤养分含量的有效措施。
-
将不同粒径团聚体的组成比例与团聚体中土壤养分进行综合考虑,可以全面反映各粒径团聚体对土壤养分的贡献率。本研究发现:不同石漠化程度土壤中,粒径>2.00 mm和粒径0.25~2.00 mm的团聚体是土壤养分的主要来源,粒径<0.25 mm的团聚体对土壤碳、氮、磷及土壤酶活性贡献率最低。虽然土壤有机碳、全氮、全磷及脲酶、β-葡萄糖苷酶、酸性磷酸酶活性在粒径>2.00 mm的团聚体中均较低,但由于土壤中粒径>2.00 mm团聚体的组成比例最高,所以粒径>2.00 mm的团聚体对土壤养分的贡献率才显示出较高值。这也进一步说明不同粒径团聚体对土壤养分及酶活性的贡献率与团聚体含量的高低有关。邱莉萍等[38]研究也发现:团聚体含量是引起团聚体养分贡献率变化的主要因素。
3.1. 石漠化程度对土壤团聚体组成及稳定性的影响
3.2. 石漠化程度对土壤团聚体碳、氮、磷及酶活性特征的影响
3.3. 不同粒径团聚体对土壤养分的贡献率
-
在岩溶区不同石漠化程度土壤粒径团聚体中,较大粒径的土壤团聚体在土壤组成上占优势,对土壤养分和酶活性的贡献率也相对较高,而较小粒径的土壤团聚体更有利于土壤营养元素和酶活性的积累,其相应的含量也更高。土壤养分及酶活性在土壤团聚体中的分布情况,可作为评价石漠化地区土壤地力的指标之一,为该地区土壤改良和植被恢复提供数据支撑和理论依据。在不同石漠化程度土壤中,淀粉酶活性变化趋势从大到小依次为潜在、轻度、重度、中度的石漠化土壤,有机碳、全氮、脲酶和β-葡萄糖苷酶活性变化趋势从大到小依次为重度、轻度、潜在、中度的石漠化土壤。研究区重度石漠化土壤养分及酶活性较高,可对重度石漠化区进行一定的植被恢复。









 DownLoad:
DownLoad:
