-
毛竹Phyllostachys edulis是中国亚热带地区种植最广泛的竹种之一,具有减缓气候变化、固碳减排的优势和潜力[1−2]。毛竹林集约经营具有增产增收等优势,但长期集约经营可能会导致毛竹林生态功能退化、土壤有机碳储量下降以及土壤碳排放加剧等问题[3]。前人研究发现:外源碳的施用可以显著提升毛竹林土壤肥力和有机碳储量[4]。农作物秸秆作为一种常见的外源碳,被广泛应用于农林生态系统中[5]。但是,秸秆直接输入土壤会引发病虫害,导致土壤酸化等问题[6−7]。相比之下,秸秆生物质炭(秸秆在高温低氧条件下热解产生的一种难以降解的富碳固态产物)具有更多的优势,如提升土壤有机碳储量、降低土壤酸度及促进作物增产等[8−9]。另外,秸秆及其生物质炭输入土壤后均会改变土壤氮素含量、微生物群落特征及氮转化过程等[10−12],从而对氮循环产生显著影响,且影响效应与外源碳类型密切相关[13−14]。氮素是毛竹生长的必需元素之一[15−16],也是其产量的限制因子[17],因此,研究不同外源碳输入对毛竹林土壤氮循环的影响具有重要意义。
硝化作用是土壤氮循环中的关键因素之一,也是旱地土壤氧化亚氮(N2O)排放的主要途径[18]。氨氧化过程是硝化作用的关键和限速步骤,由氨氧化古菌(AOA)和氨氧化细菌(AOB)共同驱动[19]。此外,复杂的有机氮需要经过微生物分泌胞外酶进行水解,才能进入下一步氮素转化过程[20]。因此,研究土壤氨氧化微生物群落及与氮循环相关的酶活性变化,有助于揭示毛竹林土壤硝化过程的生物学调控机制。
目前的研究表明:秸秆及其生物质炭的输入能够显著改变土壤中氨氧化微生物群落和氮循环相关酶活性,但研究结果存在较大差异。这可能是由于外源碳材料、土壤类型以及气候等因素的差异而产生的[21−24]。此外,现有的研究主要集中在农田土壤,对毛竹林生态系统的研究报道相对较少。因此,本研究以亚热带毛竹林为研究对象,比较玉米Zea mays秸秆及其生物质炭施用对毛竹林土壤氨氧化微生物群落、氮循环相关酶活性以及总硝化速率的影响,揭示玉米秸秆及其生物质炭输入后毛竹林土壤氮素转化的微生物学机制,为秸秆及其生物质炭在毛竹林生态系统中的合理利用提供理论基础和科学依据。
-
研究区位于浙江省杭州市临安区高虹镇泥马村(30°19′N, 119°41′E)。该地为中亚热带季风气候,年平均气温为18.1℃,年平均降水量为1764.0 mm,日照时数1950.0 h,无霜期243.0 d。土壤类型为红壤,0~20 cm土层土壤的基本理化性质:pH 4.82,有机碳19.80 g·kg−1,全氮1.88 g·kg−1,有效磷7.81 mg·kg−1,速效钾89.40 mg·kg−1,砂粒398.00 g·kg−1,粉粒321.00 g·kg−1,黏粒 281.00 g·kg−1。
-
本研究于2020—2021年在试验地毛竹林中进行。试验设置3个处理:对照(不施用玉米秸秆和生物质炭,ck)、施用玉米秸秆5 t·hm−2 (T1)、施用玉米秸秆生物质炭5 t·hm−2 (T2)。每个处理设4个重复,共计12个小区。采用随机区组设计,小区面积为100 m2 (10 m×10 m),小区之间设5 m的缓冲地带。
本研究所用的玉米秸秆及其生物质炭由南京智融联科技有限公司提供。玉米秸秆中碳和氮质量分数分别为412.3和6.6 g·kg−1。玉米秸秆生物质炭是在500 ℃限氧条件下热裂解而成,其基本理化性质如下:pH为9.24(m∶V=1∶20),含碳和氮分别为550.4 和11.9 g·kg−1,比表面积为11.3 m2·g−1。
2020年8月31日,将玉米秸秆和玉米秸秆生物质炭分别均匀施入样地,并将其翻耕至土壤表层的0~20 cm深度。于2020年11月(施用外源碳后的第3个月)和2021年9月(施用外源碳后的第12个月)进行采样。采用“五点法”采集土壤表层(0~20 cm)土样,随后将采得的土样分成2个部分,分别置于4和−80 ℃冰箱保存,用于各项指标测定。
-
土壤铵态氮(NH4 +-N)和硝态氮(NO3 −-N)质量分数用2 mol·L−1氯化钾溶液浸提并测定[25];土壤水溶性有机氮(WSON)质量分数参考LI等[26]的方法,即用蒸馏水浸提,取滤液用TOC-TN分析仪(TOC-VCPH)测定土壤水溶性总氮,另取滤液用离子色谱(ICS 1500, Thermofisher)测定溶液NH4 +-N和NO3 −-N质量分数,WSON质量分数为土壤水溶性总氮质量分数减去NH4 +-N和NO3 −-N质量分数;微生物生物量氮(MBN)采用氯仿熏蒸法测定[27]。土壤脲酶活性测定采用靛酚比色法[28];土壤蛋白酶活性测定参照LADD等[29]的方法。
-
土壤总硝化速率采用气压过程分离(BaPS)程序测定[30]。在每个小区表层(0~20 cm)采取6个土壤样品。为防止土壤水分损失,样品采集后立即密封包装。随后,将土壤样品放置在带有温度传感器的BaPS培养箱中,并将温度设置为野外实际测定的土壤温度。打开BaPS系统软件,输入样品参数,测试收集数据24 h,经Delta分析得到土壤总硝化速率,单位为μg·g−1·d−1[31]。
-
称取0.2 g新鲜土壤,使用Fast DNA Spin Kit(MP Biomedals)提取土壤总DNA,提取的DNA样品保存于−70 ℃。选用不同引物对土壤氨氧化古菌和氨氧化细菌amoA基因进行扩增(引物信息和反应条件见表1)。PCR采用AP221-02试剂盒(TransStartFastpfu DNA聚合酶,20 μL反应体系,南京诺唯赞生物科技有限公司)在ABI GeneAmp®9700型PCR仪(Applied Biosystems)中进行。使用质量分数为2%琼脂糖凝胶回收PCR产物,利用DNA凝胶回收纯化试剂盒(PCR Clean-Up Kit)回收纯化产物。
测序类型 引物名称 引物序列(5′→3′) 过程 氨氧化古菌 amoAF
amoARSTAATGGTCTGGCTTAGACG
GCGGCCATCCATCTGTATGTPCR扩增体系为20 μL。PCR扩增条件为95 ℃变性3 min,
然后进行37次热循环(95 ℃ 30 s,60 ℃退火30 s,72 ℃
45 s),最后在72 ℃停留10 min氨氧化细菌 bamoA1F
bamoA2RGGGGTTTCTACTGGTGGT
CCCCTCKGSAAAGCCTTCTTCTable 1. Primers of ammonia oxidizing archaea and ammonia-oxidizing bacteria
通过熔解曲线分析,观察琼脂糖凝胶电泳产物,证实扩增的特异性。随后用QuantiFluor™-ST蓝色荧光定量系统(Promega公司)测定氨氧化古菌和氨氧化细菌的amoA基因拷贝数。对含有克隆amoA基因的线性化质粒进行10倍梯度连续稀释,得到校准曲线。采用PCR效率为90%~110%,调整系数(R2)大于0.98的标准曲线。
-
引物组的扩增产物同样用于高通量测序。扩增产物经纯化后,使用TruSeqTMDNASample Prep Kit试剂盒构建文库,在上海美吉生物医药科技有限公司(中国)的IlluminaMiSeq平台(Illumina)上测序,获得以FASTQ格式保存的双端序列数据。测序得到的PE reads首先根据overlap关系进行拼接,同时对序列质量进行质控和过滤。根据序列首位两端的barcode和引物序列区分样品得到有效序列,并矫正序列方向,得到优化数据。利用UPARSE 11按照97%的相似度对质控拼接后的序列进行操作分类单元(operational taxonomic units,OTU)聚类并剔除嵌合体。使用RDPClassifier对比FunGene功能基因数据库进行OTU物种分类学注释,置信度阈值为70%,并在不同物种分类水平下统计每个样本的群落组成。
-
采用Excel 2010和SPSS 21.0进行数据处理和统计分析。利用单因素方差分析(one-way ANOVA)结合Tukey法检验不同处理间的差异显著性(P<0.05)。采用Pearson相关分析,分析土壤氮组分、氨氧化微生物、酶活性和总硝化速率之间的相关性。利用Canoco 5.0对土壤不同形态氮组分质量分数与氨氧化微生物群落之间的相关性进行冗余分析(redundancy analysis,RDA),并通过蒙特卡洛检验,选择出显著影响氨氧化微生物群落结构的土壤氮组分。
-
与对照相比,在试验第3个月和第12个月,秸秆处理均显著提高了土壤NH4 +-N、NO3 −-N和水溶性有机氮(P<0.05),而生物质炭处理则显著降低这些土壤氮组分的质量分数(P<0.05,图1A~C)。秸秆及其生物质炭处理均显著提高土壤微生物生物量氮(P<0.05),且在试验第3个月,秸秆及其生物质炭处理分别使土壤微生物生物量氮增加26.6%和14.4%,两者间差异显著(P<0.05),但在试验第12个月,秸秆及其生物质炭处理分别使微生物生物量氮增加17.4%和12.2%,两者间无显著差异(图1D)。
-
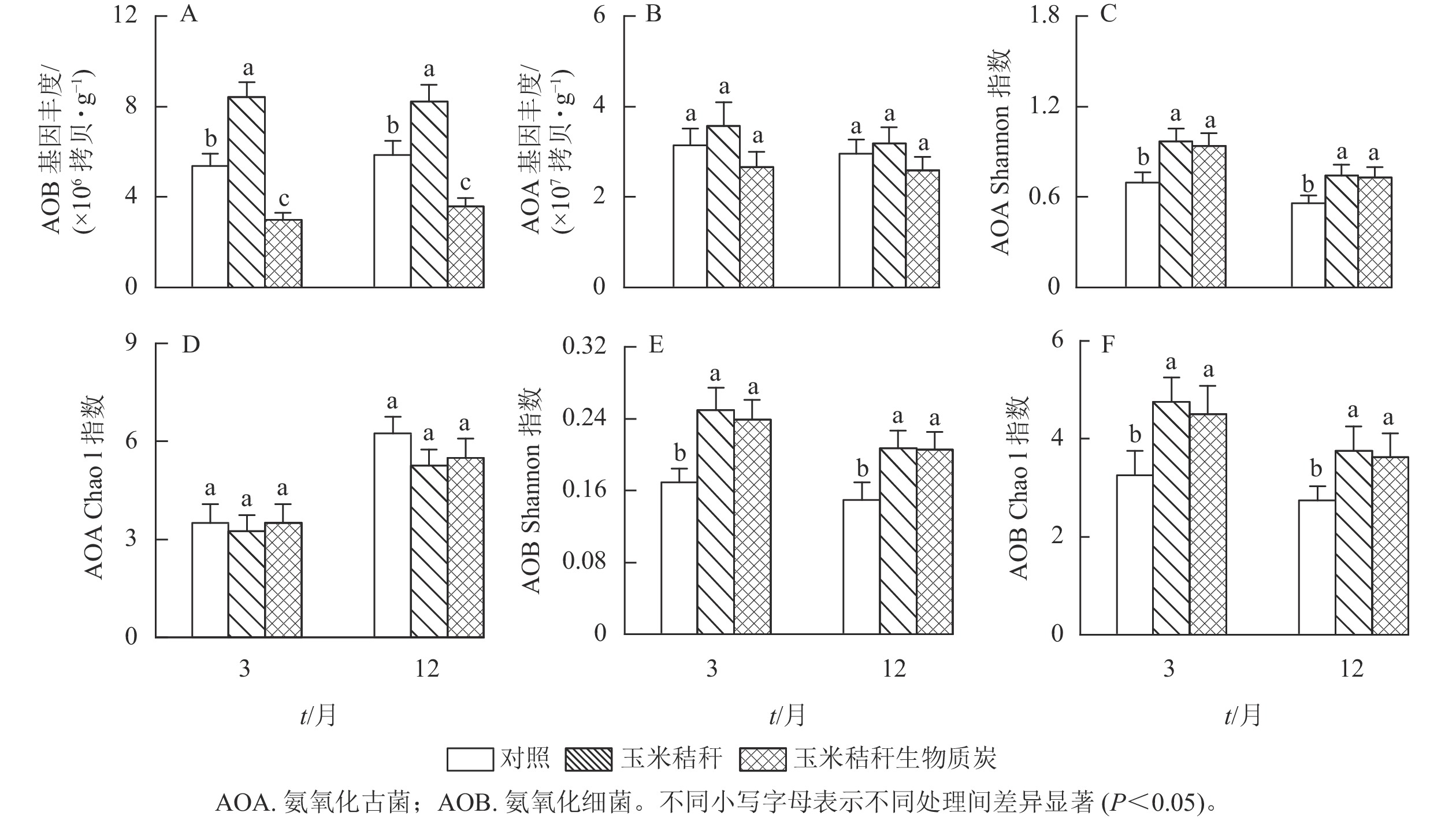
由图2A和图2B可知:与对照相比,在试验第3个月和第12个月,施用秸秆显著提高了土壤氨氧化细菌丰度(P<0.05);而生物质炭则降低了土壤氨氧化细菌丰度,降幅分别为44.6%和39.0% (P<0.05)。秸秆及其生物质炭处理对土壤氨氧化古菌丰度均无显著影响。
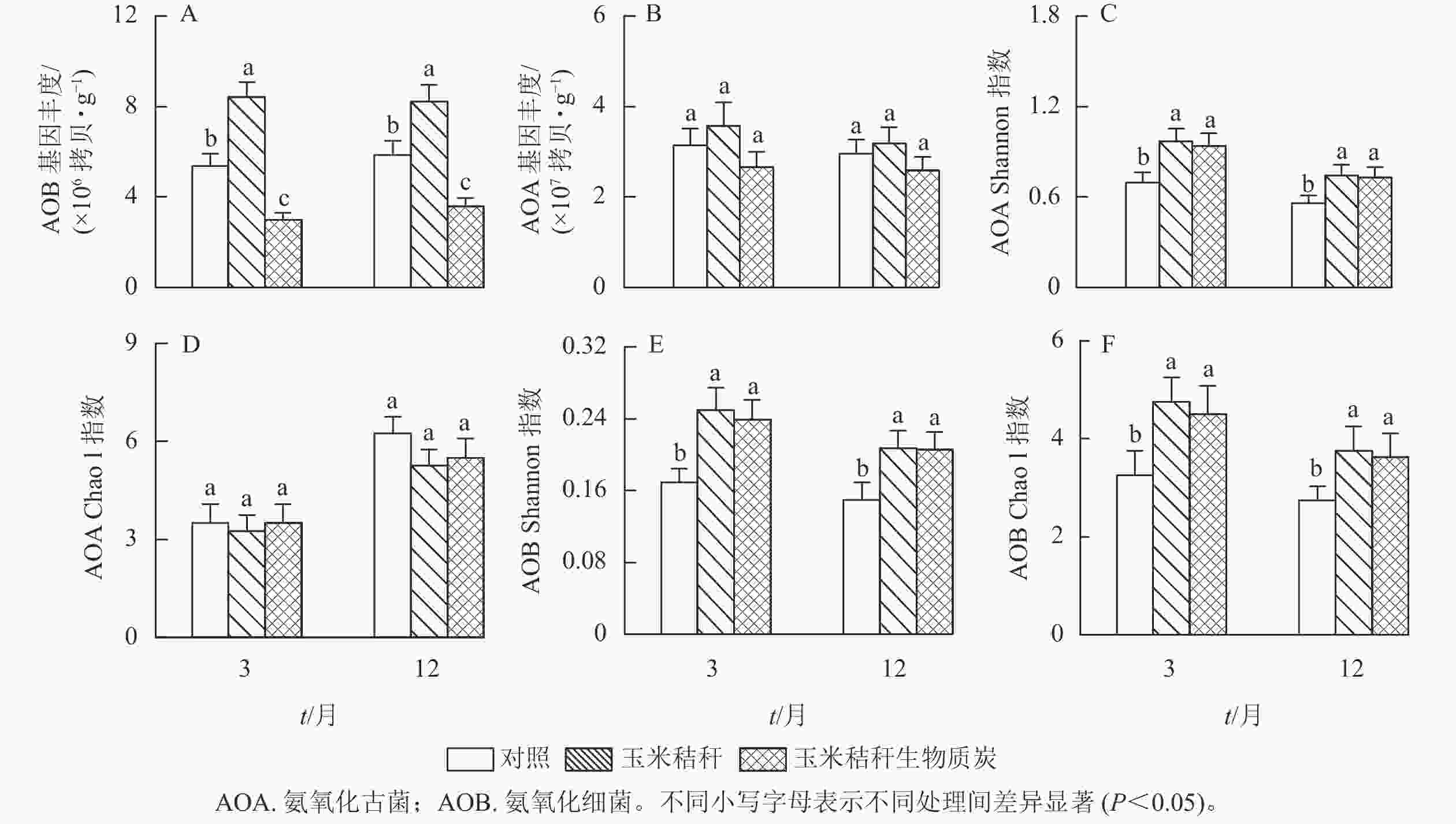
Figure 2. Effects of straw and its biochar application on the abundance and diversity index of soil AOA and AOB in a Ph. edulis forest
与对照相比,在试验第3个月和第12个月,秸秆及其生物质炭处理均显著提高了土壤氨氧化古菌群落的Shannon指数(P<0.05),而氨氧化古菌群落Chao1指数无显著变化(图2C~D);秸秆及其生物质炭处理下,土壤氨氧化细菌群落Shannon指数和Chao1指数均显著高于对照(P<0.05,图2E~F)。
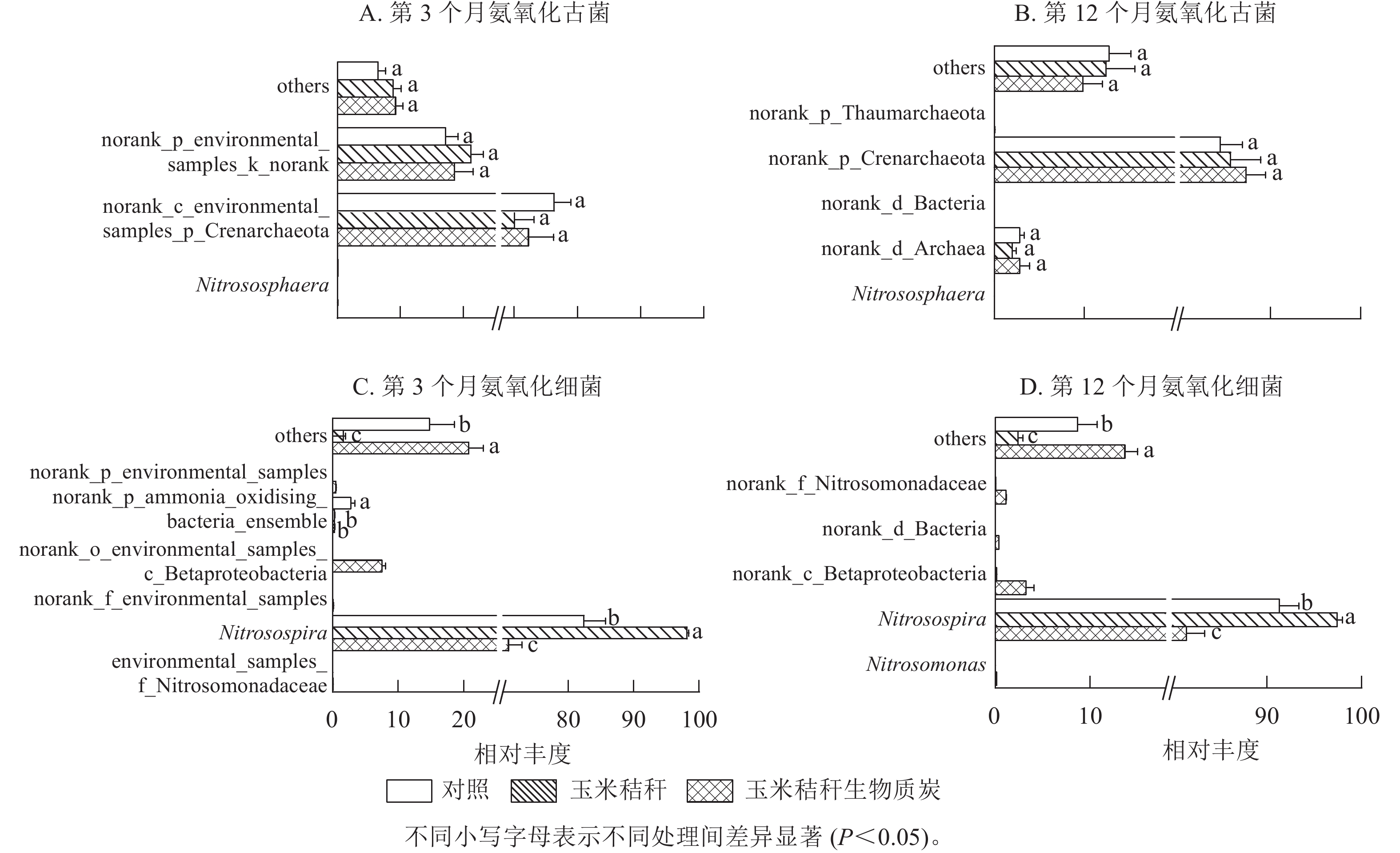
在试验第3个月,氨氧化古菌群落的优势菌属(相对丰度>1%)主要为norank_c_environmental_samples_p_Crenarchaeota和norank_p_environmental_samples_k_norank,两者相对丰度分别为70.0%~76.3%和17.2%~21.2%(图3A);在试验第12个月,氨氧化古菌群落以norank_p_Crenarchaeota和norank_d_Archaea为主,两者相对丰度分别为84.4%~87.3%和2.0%~2.8%(图3B)。在试验第3个月和第12个月,与对照相比,秸秆及其生物质炭处理均对氨氧化古菌群落组成无显著影响。
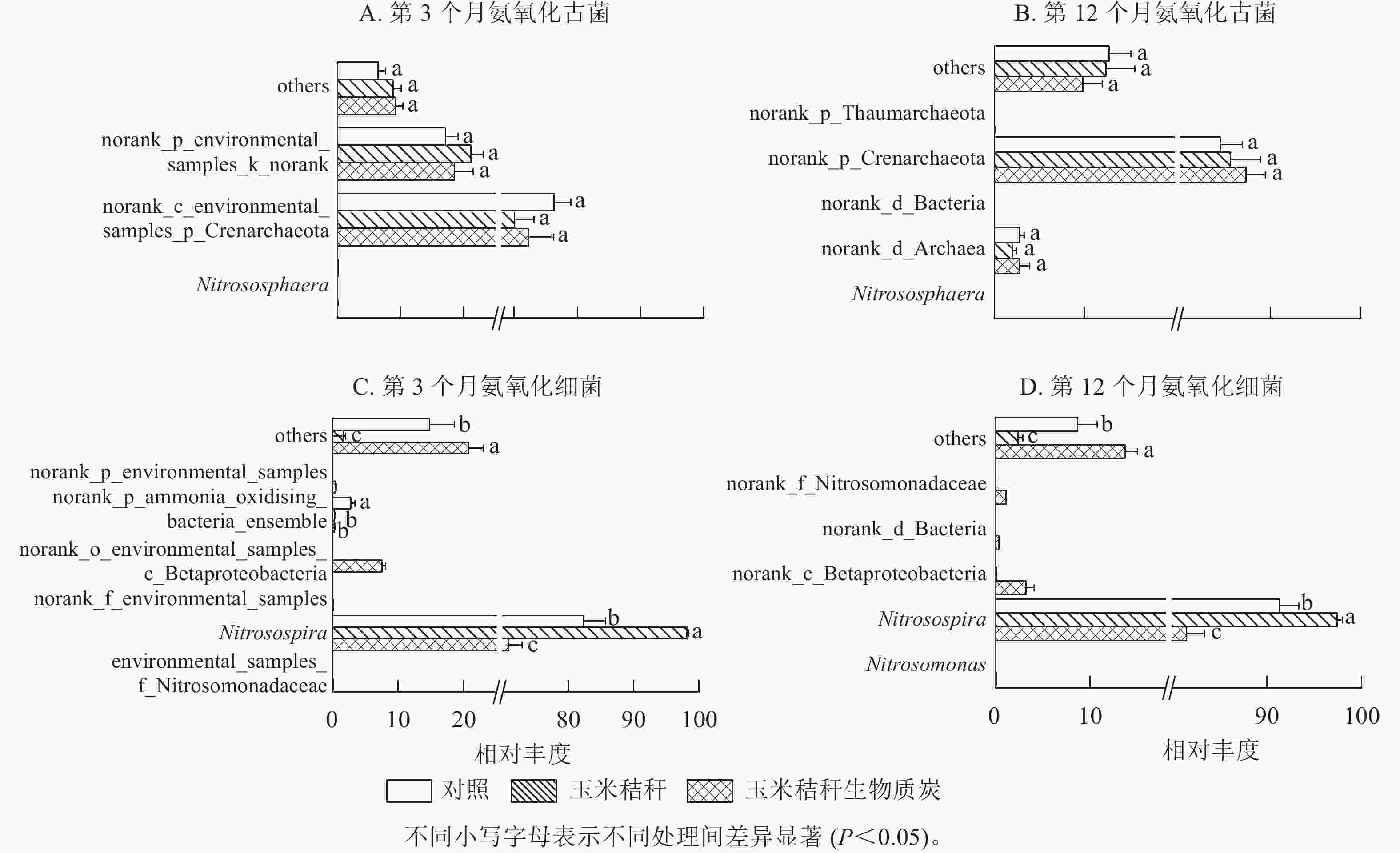
Figure 3. Effect of straw and its biochar application on the community composition of soil AOA and AOB at genus level in a Ph. edulis forest
由图3C~D可知:亚硝化螺菌属Nitrosospira在氨氧化细菌群落组成中占绝对优势。在试验第3个月,与对照相比:秸秆处理显著增加了亚硝化螺菌属的相对丰度(P<0.05),显著降低了norank_p_ammonia_oxidising_bacteria_ensemble的相对丰度(P<0.05)。生物质炭处理显著降低了亚硝化螺菌属和norank_p_ammonia_oxidising_bacteria_ensemble的相对丰度(P<0.05),增加了norank_o_environmental_samples_c_Betaproteobacteria的相对丰度。在试验第12个月,秸秆处理显著增加了亚硝化螺菌属的相对丰度(P<0.05);而生物质炭处理显著降低亚硝化螺菌属的相对丰度(P<0.05),并增加了norank_c_Betaproteobacteria和norank_f_Nitrosomonadaceae的相对丰度。
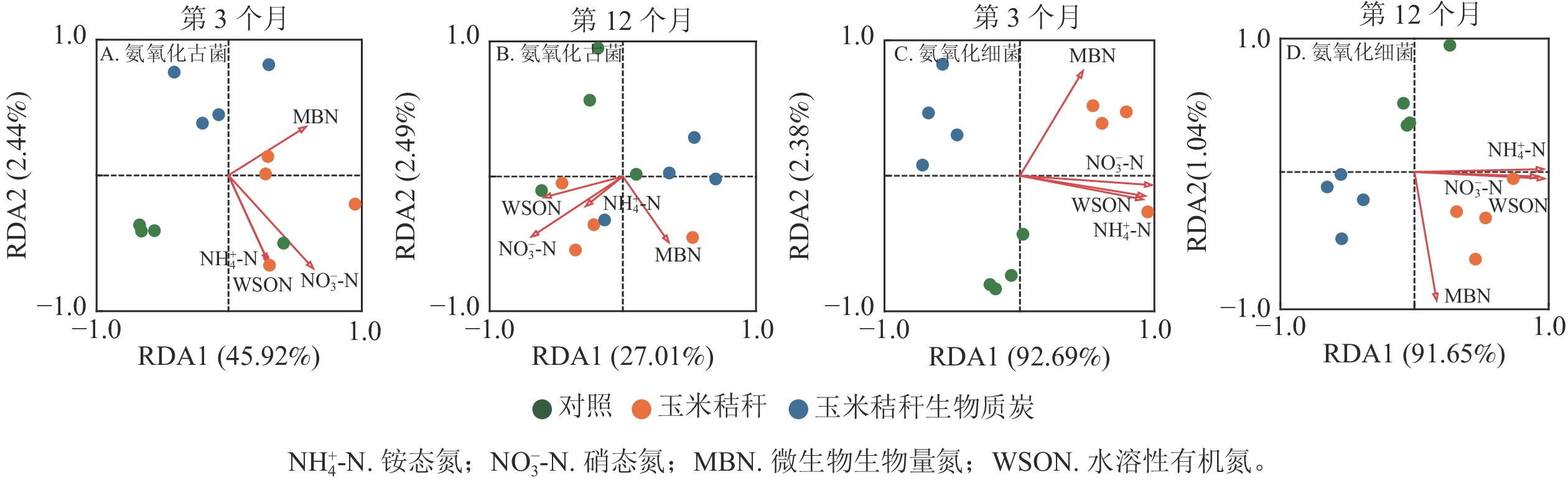
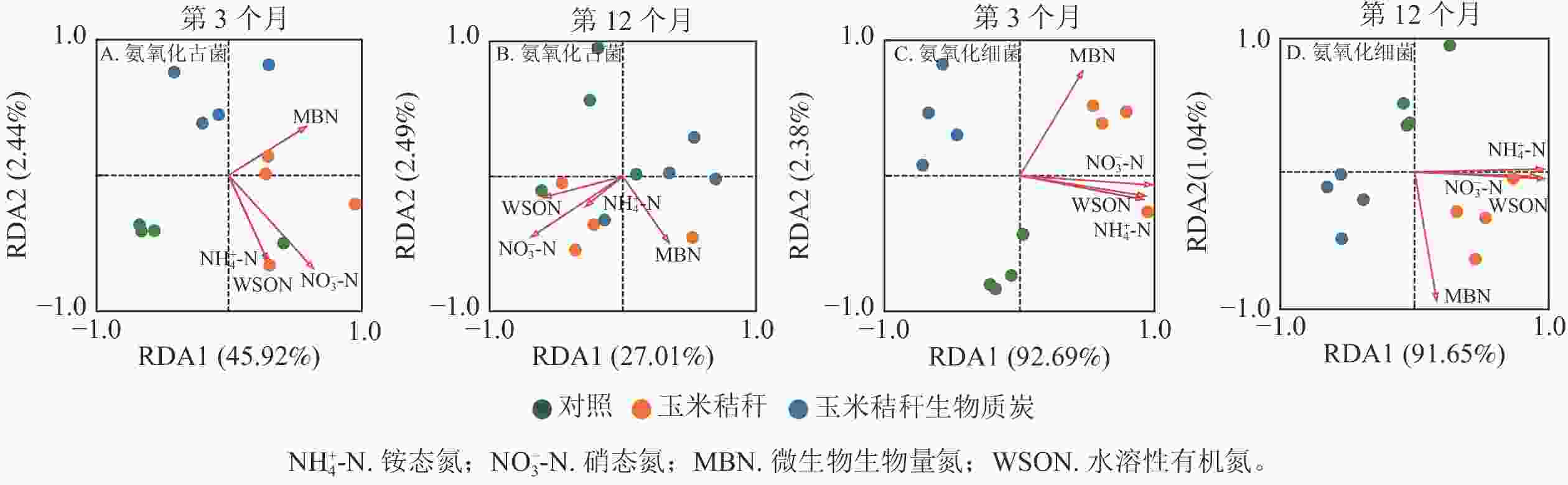
对土壤不同形态氮组分质量分数与氨氧化微生物群落之间的相关性进行冗余分析,并通过蒙特卡洛检验发现:在试验的第3个月和12个月,土壤氮组分质量分数对氨氧化古菌群落结构均无显著影响(图4A~B);在试验的第3个月,土壤NO3 −-N和微生物生物量氮为氨氧化细菌群落的关键影响因子(P<0.05,图4C),而在试验第12个月,NH4 +-N和水溶性有机氮为影响氨氧化细菌群落的主要因子(P<0.05,图4D)。
-
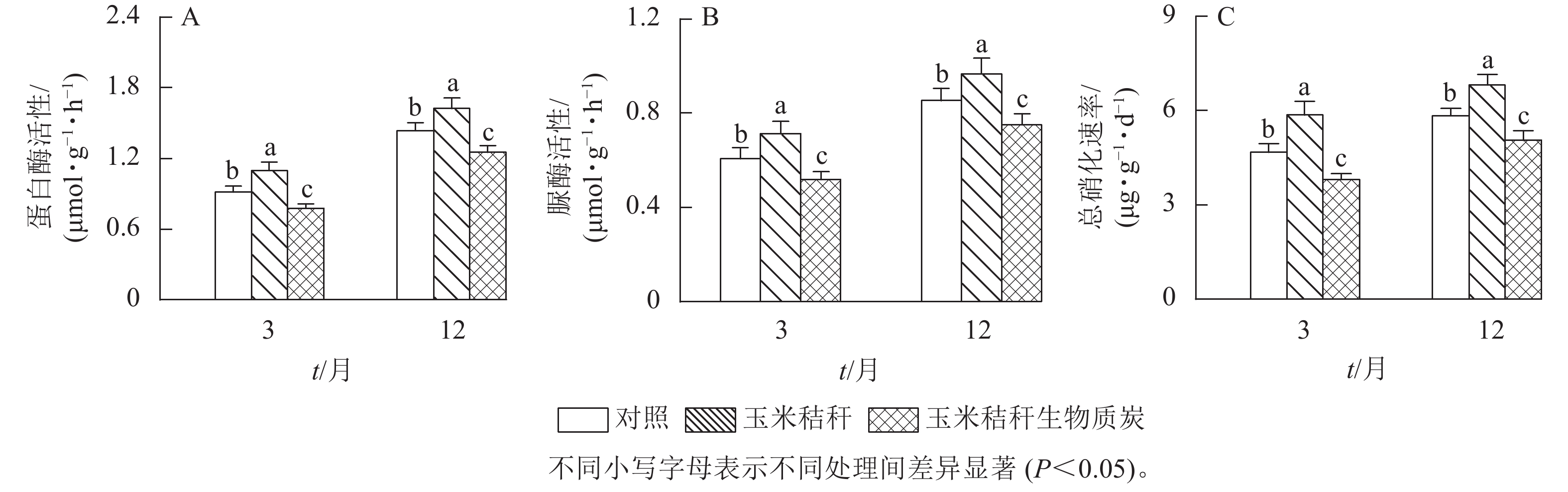
在试验第3个月和第12个月,与对照相比,秸秆处理显著提高了土壤蛋白酶和脲酶活性(P<0.05),其中,蛋白酶活性分别提高了19.9%和13.3%,脲酶活性分别提高了17.5%和13.1%;生物质炭处理则显著降低了这2种酶的活性(P<0.05),蛋白酶活性的降幅分别为15.2%和12.7%,脲酶活性的降幅分别为14.6%和12.0% (图5A~B)。在试验第3个月和第12个月,与对照相比,秸秆处理使土壤总硝化速率提高了25.1%和16.6% (P<0.05),生物质炭处理则显著降低了土壤总硝化速率(P<0.05),降幅分别为18.9%和13.6% (图5C)。
-
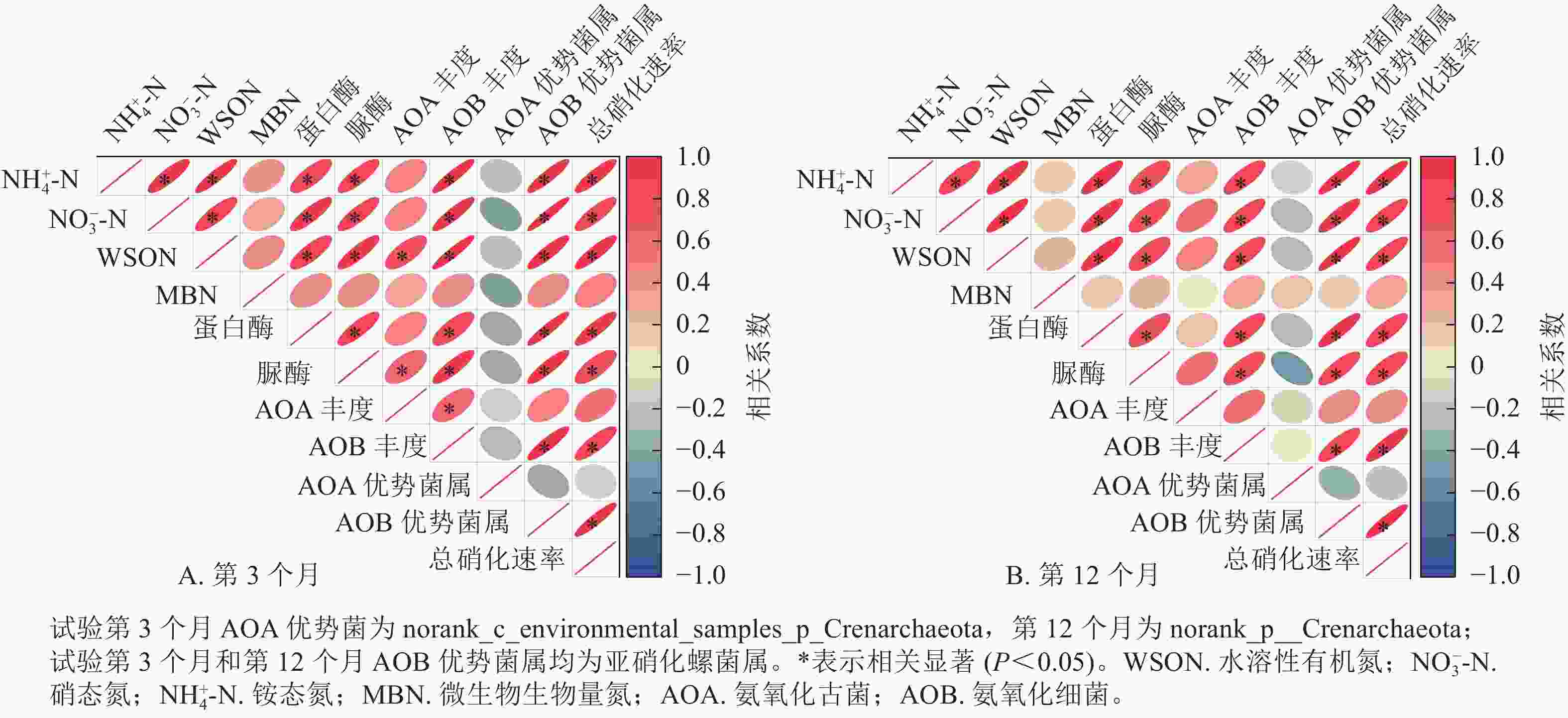
相关性分析表明(图6):除在试验第3个月,氨氧化古菌丰度与土壤水溶性有机氮质量分数呈显著正相关(P<0.05)之外,氨氧化古菌丰度及其优势菌的相对丰度与土壤氮组分质量分数、总硝化速率之间无显著相关。在第3个月和第12个月,土壤氨氧化细菌丰度及其优势菌属亚硝化螺菌属的相对丰度、蛋白酶活性和脲酶活性均与NH4 +-N、NO3 −-N和水溶性有机氮以及总硝化速率呈显著正相关(P<0.05);土壤NH4 +-N、NO3 −-N和水溶性有机氮也均与总硝化速率呈显著正相关(P<0.05)。
-
土壤氨氧化古菌和氨氧化细菌是土壤硝化作用的关键微生物群体,它们对环境变化的响应存在差异[32]。本研究发现:毛竹林土壤氨氧化细菌丰度及其群落结构受秸秆及生物质炭输入显著影响,而土壤氨氧化古菌群落变化受到的影响较小。这可能是由于土壤氨氧化细菌的生态位小于氨氧化古菌,即其在土壤中占据的资源和空间范围较窄;而土壤氨氧化古菌具有更广泛的生态位,能够在不同的温度、pH、氧气和氮素等条件下生存和活动[33−34]。因此,土壤氨氧化细菌对环境变化的适应能力相对较低,对管理措施的响应更加敏感。另外,本研究也发现:土壤中不同形态氮组分对氨氧化细菌丰度及群落结构产生了显著影响,而对氨氧化古菌丰度和群落结构无显著影响。这也进一步证实了土壤氨氧化细菌对环境变化的响应更加敏感。这可能是由于氨氧化古菌和氨氧化细菌对不同形态的氮素反应能力存在差异,氨氧化细菌对于土壤中氮素的利用和转化具有更高的依赖性,因此在面对环境变化的时候更易受影响[35]。
本研究结果显示:玉米秸秆处理显著提高了土壤氨氧化细菌丰度,这可能是因为秸秆输入提供了充足的底物,从而促使氨氧化细菌生长,这与以往大多数研究结果一致[36−37]。此外,秸秆的施用还改变了土壤氨氧化细菌群落的结构,显著增加了氨氧化细菌优势菌属亚硝化螺菌属的相对丰度。与秸秆处理相反,玉米秸秆生物质炭输入降低了土壤氨氧化细菌丰度,并减少了亚硝化螺菌属的相对丰度。这可能是因为生物质炭具有较高的比表面积和孔隙结构,对土壤氮素具有吸附作用[38],或者其对硝化作用具有抑制作用,减少了土壤中硝酸盐的生成,降低了土壤中可溶性氮组分质量分数[39]。这导致氨氧化细菌生长所需的氮素减少,降低了氨氧化细菌丰度[40]。另外,生物质炭本身的物质组成可能包含硝化抑制剂α-松萜或酚类物质。这些化合物可能与土壤中的氨氧化细菌相互作用,干扰了其正常的代谢活动,导致氨氧化细菌丰度降低[41]。亚硝化螺菌属主要利用土壤中的氨氮和亚硝酸盐进行生长和代谢,并通过氨氧化和亚硝酸氧化将这些无机氮化合物转化为硝酸盐[42]。因此,当生物质炭输入使土壤中的无机氮降低时,亚硝化螺菌属可能会面临氮源供应的不足,不利于其生长和繁殖。此外,生物质炭输入还提高了土壤氨氧化细菌群落的多样性,这使得氨氧化细菌属间的竞争加剧,从而对氨氧化细菌优势菌属的生长产生抑制作用[43]。
-
土壤蛋白酶和脲酶是参与土壤氮循环的主要酶系,其活性与土壤氮素转化强度和供氮能力紧密相关[44−45]。本研究中,秸秆输入对土壤蛋白酶和脲酶活性均有刺激作用,导致土壤酶活性显著提高。这可能是由于秸秆输入提高了土壤有机质质量分数,为微生物提供了丰富的营养物质,促使微生物生长,提高了土壤酶活性[45−46]。相反,秸秆生物质炭的输入会对土壤蛋白酶和脲酶活性产生负面影响,这可能是由于生物质炭对反应底物和酶分子具有吸附作用。这种吸附作用会限制酶与底物接触,阻碍酶促反应,导致土壤酶活性降低[47−48]。此外,生物质炭可能会占据酶的活性位点,使酶分子无法有效地与底物结合,降低了酶的活性[49]。
土壤酶活性与不同氮素组分的水平紧密相关[50−51]。蛋白酶和脲酶活性的提高有利于促进土壤有机氮分解为铵态氮[52]。本研究的相关性分析表明:毛竹林土壤蛋白酶和脲酶活性与土壤NH4 +-N、NO3 −-N和水溶性有机氮呈正相关,其中,土壤水溶性有机氮质量分数与酶活性之间的相关性最高。这与前人研究结果相符[53],说明水溶性有机氮质量分数是影响毛竹林土壤酶活性的主要因素之一。
秸秆输入显著提高了毛竹林土壤总硝化速率,而秸秆生物质炭输入则显著降低了土壤总硝化速率。前者可能是由于秸秆中的有机物质激活和增强了酶的活性,也为土壤硝化细菌提供底物,促进其生长和代谢活动,从而促进硝化作用[54−55]。后者则可能是由于生物质炭对无机氮的吸附作用以及对土壤酶活性和硝化细菌的抑制作用,导致土壤硝化作用削弱[56]。本研究还发现:氨氧化细菌在毛竹林土壤硝化作用中起主要驱动作用,而其中亚硝化螺菌属是优势菌属,其相对丰度与土壤总硝化速率呈显著正相关。前人研究结果也发现:土壤硝化作用的削弱归因于亚硝化螺菌属相对丰度的降低[57],表明亚硝化螺菌属是土壤硝化作用的关键属[43]。此外,土壤蛋白酶和脲酶活性也与总硝化速率呈显著正相关,这说明增强土壤酶活性有利于促进土壤硝化作用。
综上所述,与玉米秸秆输入相比,玉米秸秆生物质炭降低了土壤氨氧化细菌丰度及优势菌属亚硝化螺菌属的相对丰度,同时削弱了土壤中蛋白酶和脲酶的活性,因而,抑制了毛竹林土壤硝化作用。
-
玉米秸秆及其生物质炭通过影响毛竹林土壤氮组分质量分数,对氨氧化细菌群落及氮循环相关酶活性产生影响,进而调节土壤硝化作用。秸秆输入会刺激土壤硝化作用,而生物质炭输入则会抑制土壤硝化作用。相较于秸秆直接输入,将秸秆生物质炭输入毛竹林土壤能够显著抑制土壤硝化作用,因此可以被视为减少亚热带森林土壤氧化亚氮排放和氮素损失的一种有效途径。
Effects of straw and its biochar application on soil ammonia-oxidizing microorganisms and N cycling related enzyme activities in a Phyllostachys edulis forest
doi: 10.11833/j.issn.2095-0756.20230388
- Received Date: 2023-07-04
- Accepted Date: 2023-09-26
- Rev Recd Date: 2023-09-20
- Available Online: 2024-01-19
- Publish Date: 2024-02-20
-
Key words:
- biochar /
- Phyllostachys edulis forest soil /
- ammonia oxidizing microorganisms /
- soil enzyme activity /
- nitrification
Abstract:
| Citation: | PAN Lixia, JIANG Zhenhui, ZHANG Wenyi, et al. Effects of straw and its biochar application on soil ammonia-oxidizing microorganisms and N cycling related enzyme activities in a Phyllostachys edulis forest[J]. Journal of Zhejiang A&F University, 2024, 41(1): 1-11. DOI: 10.11833/j.issn.2095-0756.20230388 |







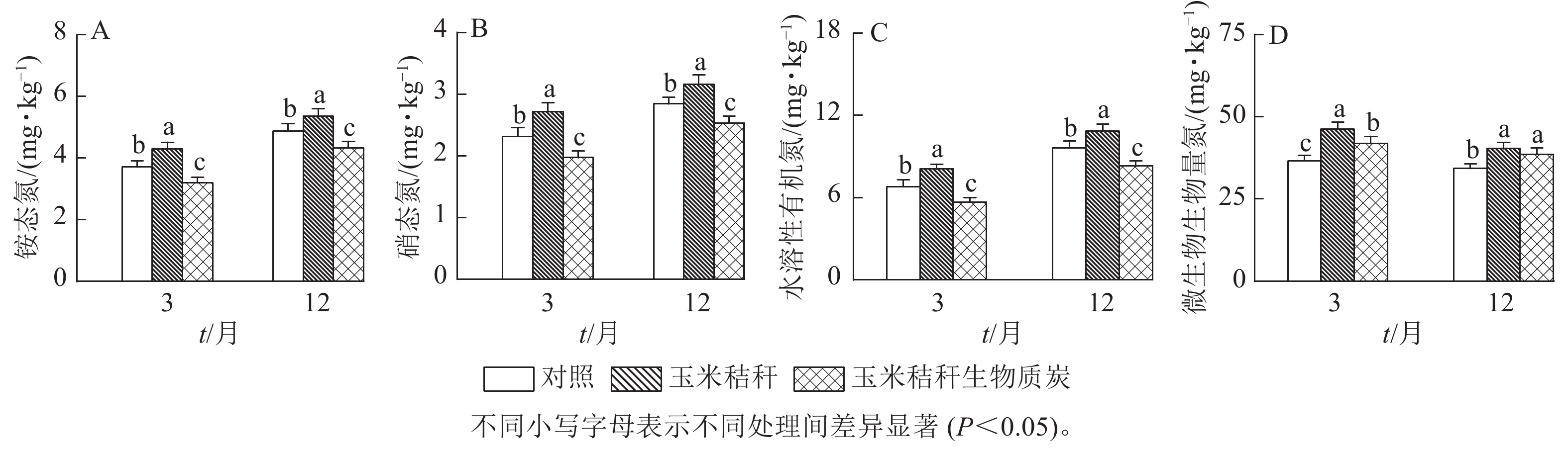



 DownLoad:
DownLoad: