-
红松Pinus koraiensis是构成东北温带森林顶极群落红松阔叶林的建群种,也是东北林区人工栽培和研究历史最长、经济和生态价值最大的珍贵树种[1]。在天然阔叶红松林中,红松不仅是乔木层的主要树种,还对维持森林生态系统的稳定性和生物多样性发挥着重要作用[2]。然而,红松本身的生物学特性,如繁殖周期长、扦插繁殖系数低以及天敌危害等原因,导致红松优良品种的选育进程较为滞后[3]。体细胞胚胎发生是解决优质种源稀缺问题的有效方法之一[4−5]。增殖阶段的胚性愈伤组织是体细胞胚胎生理生化研究、遗传改良和优良种质育苗等一系列实验的基础材料[6]。培养基的条件和外界环境会影响胚性愈伤组织的生长状况及其胚性特征的保持[7−9]。
多胺(PAs)中的腐胺(Put)、精胺(Spm)、亚精胺(Spd)和热精胺(Tspm)等是一类脂肪族小分子,在植物中含量丰富,并且可以与核酸、酶、蛋白质和带负电荷的细胞功能基团结合,参与DNA、RNA和蛋白质代谢的调控[10]。多胺不仅在体细胞胚胎发育中发挥着重要作用,还参与植物衰老、芽分化以及生物和非生物胁迫反应的调控[11]。在植物体细胞胚胎发生过程中,内源多胺的含量会发生不同程度的变化[12]。SATHISH等[13]研究发现:外源多胺可使甘蔗Saccharum spp.的体细胞胚胎发生率提高2倍以上。邢更妹等[14]研究表明:维持一定水平的多胺对枸杞Lycium barbarum的体细胞胚发生至关重要。在对棉花Gossypium hirsutum体细胞胚胎发生时期的培养物进行多胺测定时发现:胚性愈伤组织形成后多胺含量显著上升[15]。尽管已有研究在棉花等植物中揭示了多胺对体细胞胚胎发生的调控作用,但多胺在红松体细胞胚胎发生过程中的功能仍不明确。目前,关于外源多胺对红松胚性愈伤组织增殖效应及其生理机制尚缺乏研究。鉴于此,本研究以红松胚性愈伤组织为材料,通过在增殖培养基中添加不同质量浓度的多胺,探究外源多胺对红松胚性愈伤组织增殖的影响,旨在为优化红松体细胞胚胎发生体系提供科学依据。
-
前期以红松未成熟合子胚为材料,诱导产生胚性愈伤组织并超低温保存。诱导培养基为改良的利特维固体培养基(mLV),并添加2.0 mg·L−1 1-萘乙酸(NAA)、1.5 mg·L−1 6-苄氨基嘌呤(6-BA)、0.5 g·L−1酸水解酪蛋白、30.0 g·L−1蔗糖和4.0 g·L−1结冷胶,灭菌前将培养基pH调至5.8,灭菌后在超净工作台中加入0.5 g·L−1谷氨酰胺。将超低温保存的胚性愈伤组织接种到增殖培养基上复苏,初始用滤纸隔离愈伤组织与培养基,第3天更换1次滤纸和培养基,后续按正常继代周期更换培养皿(无需滤纸)。经过2个增殖周期的观察(1个周期为14 d),筛选出增殖状态优良的3个胚性细胞系(编号L-1、L-2、L-3,正常状态为松软且表面有蓬松丝状物)。增殖培养基选用mLV固体培养基[16],添加0.5 mg·L−1 6-BA、2.0 mg·L−1 2,4-二氯苯氧乙酸(2,4-D)、0.5 g·L−1酸水解酪蛋白、30.0 g·L−1蔗糖和4 g·L−1结冷胶,灭菌前将pH调至5.8,灭菌后在超净工作台中加入0.5 g·L−1谷氨酰胺及不同质量浓度(0、5、10、20、30、40、50、60 mg·L−1)的多胺,包括腐胺、精胺、亚精胺分别添加到培养基中,于温度为(23±2) ℃条件下暗培养,每组实验重复3次。
-
在超净工作台中用镊子分别夹取并称量3个胚性细胞系各3簇愈伤组织,每簇鲜质量约0.2 g,添加到培养基上。每组培养皿处理进行3个重复,增殖培养14 d后再次称取愈伤组织鲜质量,并计算14 d时的增殖率(V)。V=(G1−G0)/G0×100%。其中:G0为初始愈伤组织鲜质量,G1为增殖14 d后愈伤组织鲜质量。
-
第14天时,在外源添加不同多胺处理的细胞系中,选择增殖率较好的3个细胞系愈伤组织为材料进行生理指标测定,以不添加多胺的愈伤组织作为对照(ck)。其中,过氧化氢(H2O2)质量摩尔浓度和超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)等抗氧化酶活性均使用试剂盒(苏州科铭)测定;吲哚-3-乙酸(IAA)、脱落酸(ABA)和乙烯(ETH)按照LI等[17]的方法测定;腐胺、亚精胺和精胺按照DUTRA等[18]的方法测定,并使用酶联免疫吸附试剂盒(上海酶联生物)检测。红松愈伤组织生长状态使用光学显微镜拍摄记录。每个实验均重复3次。
-
使用SPSS软件进行单因素方差分析(one-way ANOVA)和邓肯多重比较(Duncan,α=0.05),并用Origin 2022软件绘图。
-
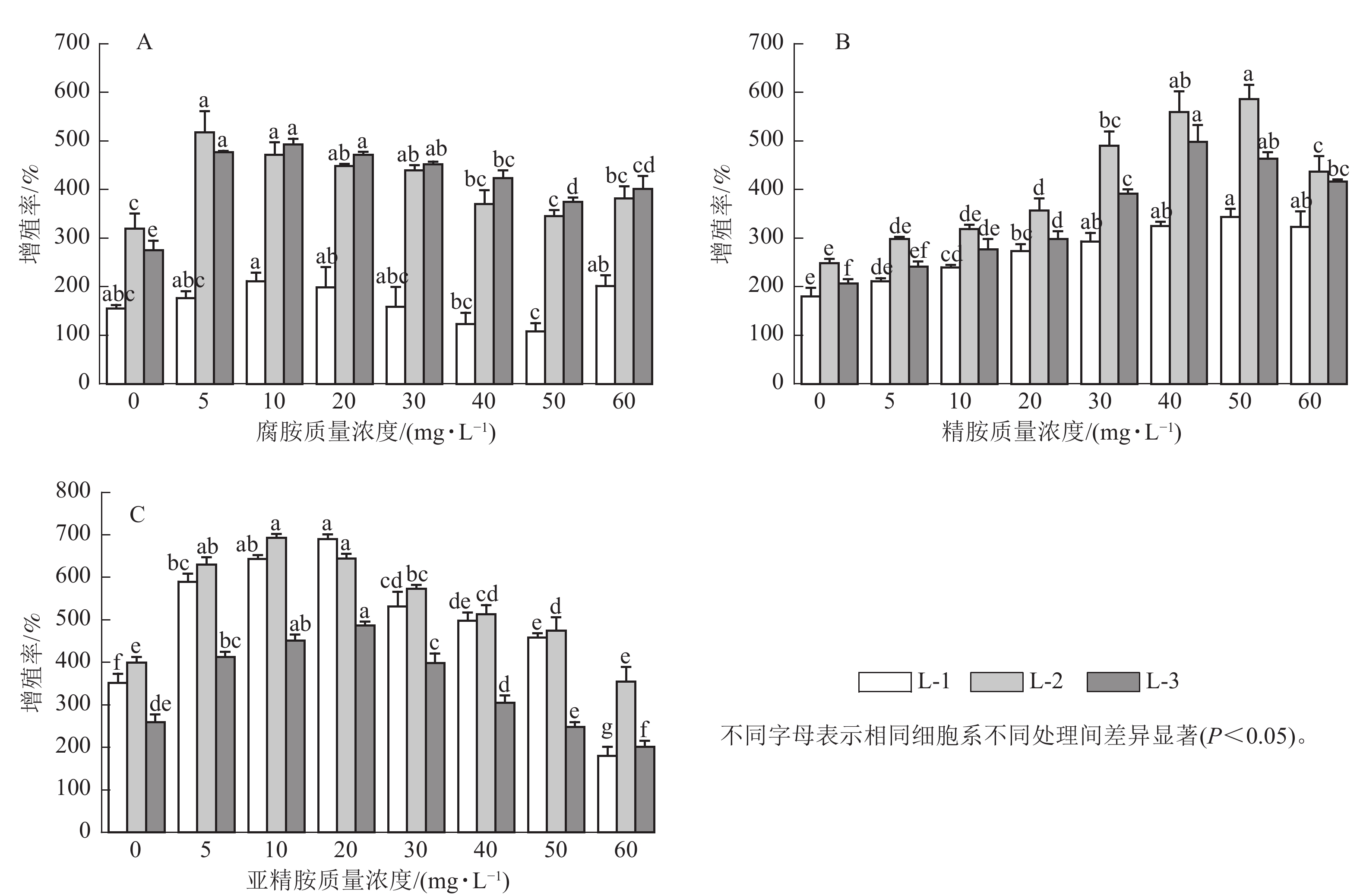
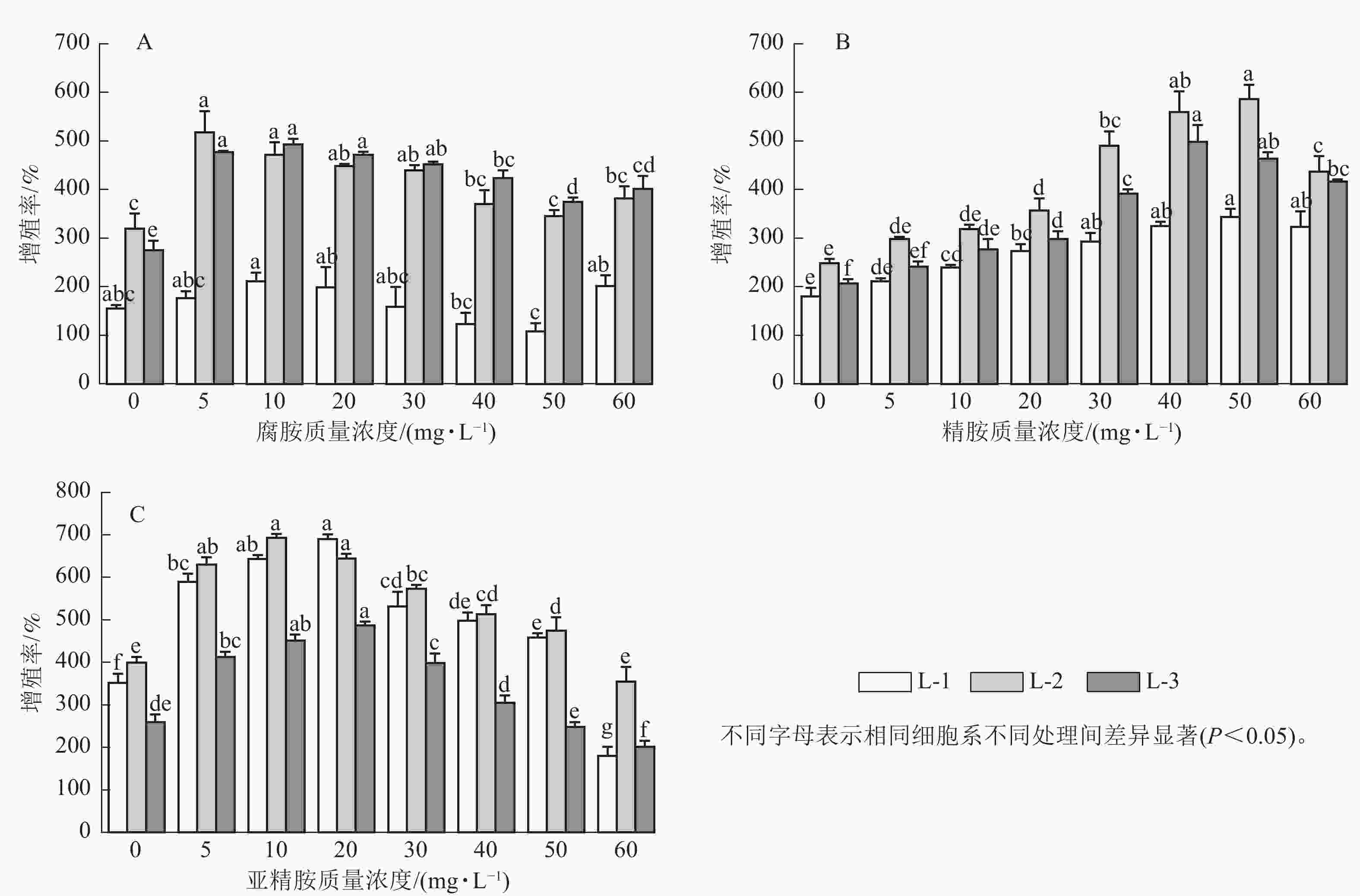
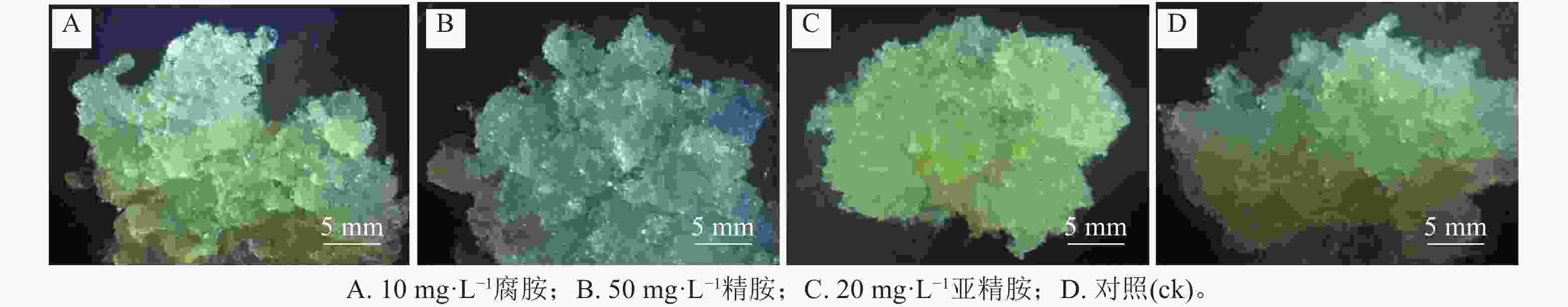
随着多胺质量浓度的升高,3个细胞系愈伤组织增殖率均呈先上升后下降的趋势。其中,外源添加5~30 mg·L−1腐胺显著促进了L-2和L-3细胞系的增殖(P<0.05),随着腐胺质量浓度的增加,愈伤组织增殖率逐渐下降,但在60 mg·L−1腐胺处理下略有回升。针对L-1细胞系,所有处理均未达到显著水平,但外源添加10 mg·L−1腐胺对其增殖有一定的促进效果。说明5~30 mg·L−1腐胺是红松愈伤细胞系适宜的质量浓度,在此质量浓度下,3个细胞系的增殖率为158.33%~518.33%,较对照增加了2.22%~79.27% (图1A)。相对于对照,外源添加10~60 mg·L−1精胺显著促进L-1和L-3细胞系的增殖(P<0.05),外源20~60 mg·L−1精胺显著促进L-2细胞系的增殖(P<0.05),其中40~50 mg·L−1精胺是3个细胞系处理的最优质量浓度,在此质量浓度下,3个细胞系的增殖率为325.00%~586.67%,相比对照增殖效果提升了80.56%~141.12% (图1B)。相对于对照,外源添加5~50 mg·L−1亚精胺显著促进L-1和L-2细胞系的增殖(P<0.05),外源添加5~30 mg·L−1亚精胺显著促进L-3细胞系的增殖(P<0.05),其中10~20 mg·L−1亚精胺是3个细胞系的最优处理,在此质量浓度下,3个细胞系的增殖率为451.67%~693.33%,相比对照增殖效果提升了61.25%~96.21% (图1C)。可见,不同类型的多胺对红松愈伤组织的增殖效率具有不同程度的影响。其中,外源添加10 mg·L−1腐胺、50 mg·L−1精胺以及20 mg·L−1亚精胺均能够显著促进不同红松细胞系中愈伤组织的增殖(P<0.05)。因此,在后续的研究中,将重点围绕外源添加的10 mg·L−1腐胺、50 mg·L−1精胺以及20 mg·L−1亚精胺进行分析。显微观察发现:多胺处理后的红松胚性愈伤组织明亮清透、状态松软,且表面有蓬松丝状物(图2),表明外源添加10 mg·L−1腐胺、50 mg·L−1精胺以及20 mg·L−1亚精胺能够较好地促进愈伤组织的生长。
-
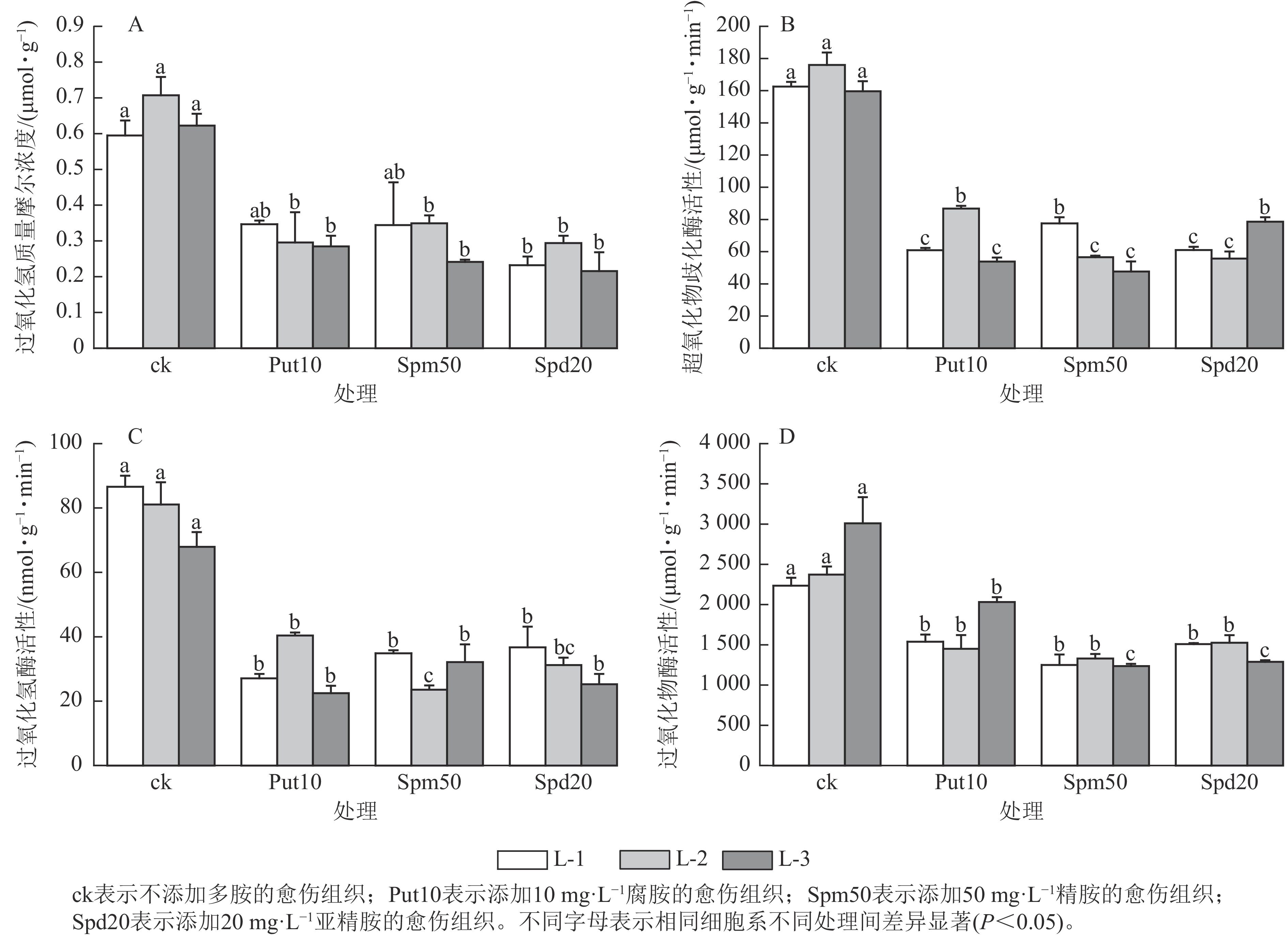
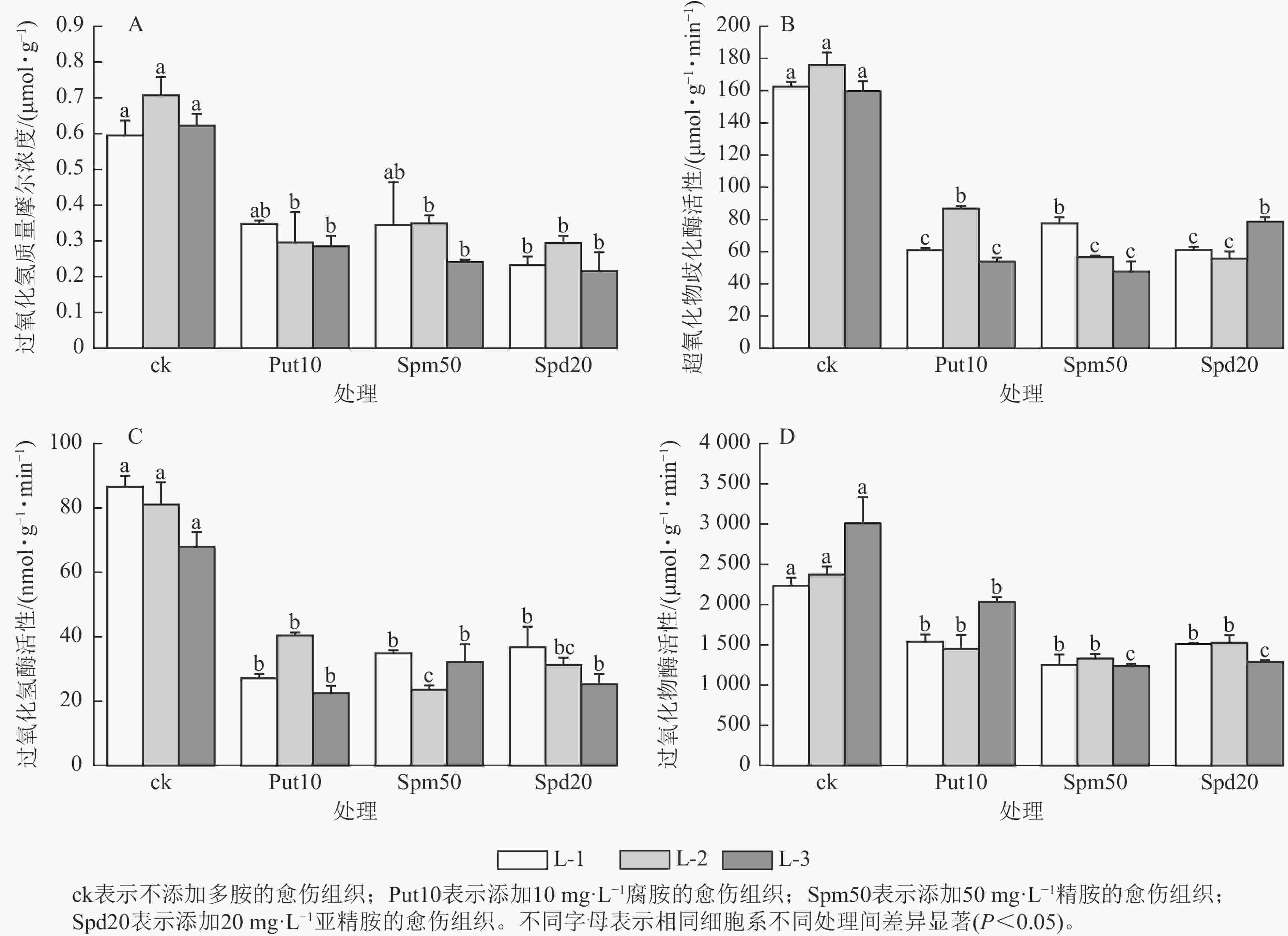
从图3A可见:与对照相比,外源添加10 mg·L−1腐胺对L-2和L-3细胞系的过氧化氢质量摩尔浓度有显著影响(P<0.05),相比ck分别降低了59.20%和54.80%。外源添加50 mg·L−1精胺对L-2和L-3细胞系的过氧化氢质量摩尔浓度影响显著(P<0.05),相比ck分别降低了50.70%和61.30%。此外,外源添加20 mg·L−1亚精胺对L-1、L-2和L-3细胞系的过氧化氢质量摩尔浓度影响显著(P<0.05),相比ck分别降低了61.00%、59.20%和66.70%。
-
在超氧化物歧化酶活性变化方面,当添加10 mg·L−1腐胺时,L-1、L-2和L-3细胞系超氧化物歧化酶活性分别为60.90、86.70和53.90 μmol·g−1·min−1,与ck相比分别下降了62.54%、50.70%和66.20%;添加50 mg·L−1精胺时,L-1、L-2和L-3细胞系超氧化物歧化酶活性分别为77.56、56.58和47.69 μmol·g−1·min−1,与ck相比分别下降了52.30%、67.80%和70.10%;添加20 mg·L−1亚精胺时,L-1、L-2和L-3细胞系超氧化物歧化酶活性与ck相比下降了50.76%~68.30% (图3B)。在过氧化氢酶活性变化方面,外源添加10 mg·L−1腐胺使3个胚性愈伤组织的过氧化氢酶活性相较于ck显著下降了40.69%~59.51% (P<0.05);添加50 mg·L−1外源精胺使愈伤组织的过氧化氢酶活性显著降低了51.70%~57.50% (P<0.05);添加20 mg·L−1外源亚精胺对L-1和L-3细胞系的过氧化氢酶活性影响显著(P<0.05),过氧化氢酶活性分别降至36.72和25.20 nmol·g−1·min−1,与ck相比分别下降了57.60%和63.83% (图3C)。在过氧化物酶活性变化方面,当添加10 mg·L−1腐胺时,3个细胞系过氧化物酶活性相较于对照下降了31.14%~38.90%;添加50 mg·L−1精胺时,3个胚性愈伤组织的过氧化物酶活性降低了43.93%~58.94%;添加20 mg·L−1亚精胺时同样降低了愈伤组织的过氧化物酶活性,下降了32.47%~57.17% (图3D)。
-
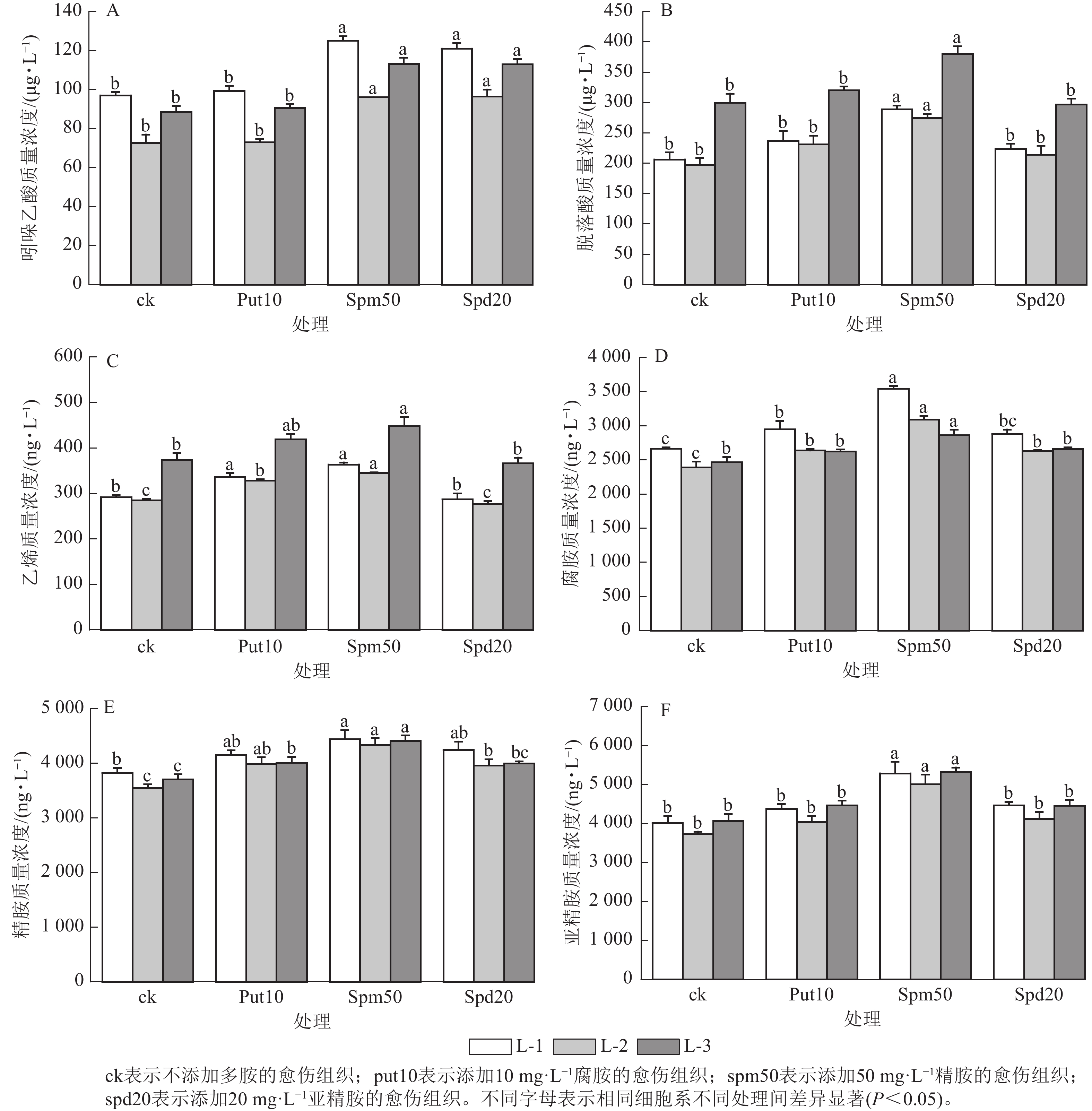
在不同多胺处理下,不同细胞系激素质量浓度的变化趋势表现出较高的一致性。当外源添加50 mg·L−1精胺和20 mg·L−1亚精胺时,均显著提高了3个细胞系内源吲哚乙酸质量浓度(P<0.05)(图4A);而添加10 mg·L−1腐胺对3个细胞系吲哚乙酸质量浓度的变化无显著影响。
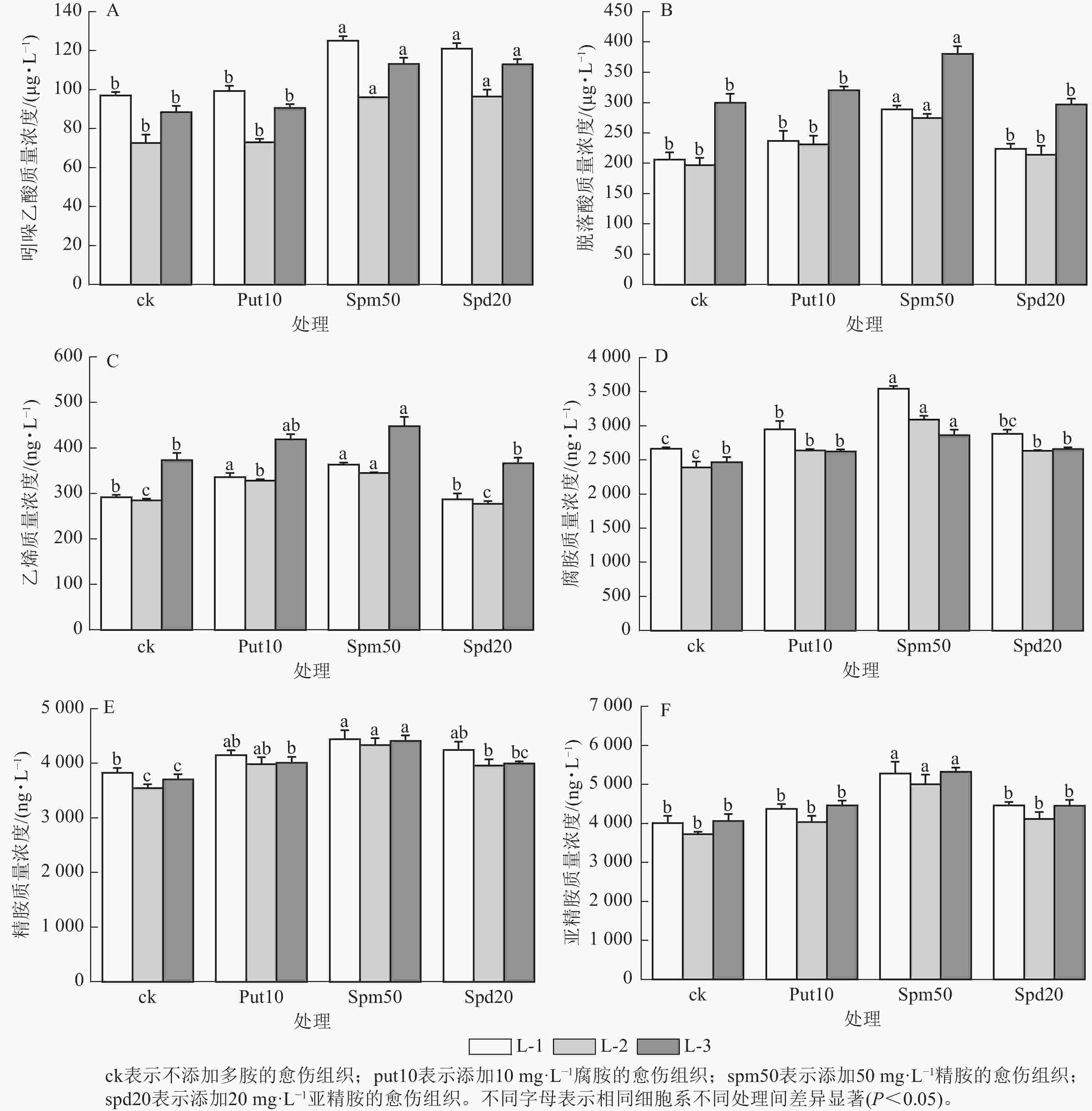
Figure 4. Effect of exogenous polyamines on the hormone and polyamine content of embryonic calli of P. koraiensis
外源添加50 mg·L−1精胺显著提高了L-1、L-2和L-3细胞系中的脱落酸质量浓度(P<0.05),与ck相比,分别提高了39.99%,39.65%和26.92% (图4B);而外源添加10 mg·L−1腐胺和20 mg·L−1亚精胺对3个细胞系脱落酸质量浓度无显著影响。
外源添加10 mg·L−1腐胺和50 mg·L−1精胺显著提高了L-1、L-2和L-3细胞系的乙烯质量浓度(P<0.05)(图4C)。而添加20 mg·L−1亚精胺对3个细胞系的乙烯质量浓度无显著影响。
-
外源添加10 mg·L−1腐胺和20 mg·L−1亚精胺能显著提升L-1、L-2细胞系的内源腐胺质量浓度(P<0.05),而对L-3细胞系的内源腐胺质量浓度无显著影响。当外源添加50 mg·L−1精胺时,L-1、L-2和L-3个细胞系的内源腐胺质量浓度达到最高,分别为3 544.68、3 095.13和2 864.58 ng·L−1,与ck相比分别增加了33.08%、29.40%和16.12% (图4D)。
外源添加10 mg·L−1腐胺和20 mg·L−1亚精胺能显著提高了L-2细胞系的内源精胺质量浓度(P<0.05),分别为3 988.23和3 961.29 ng·L−1,与ck相比分别增加了12.35%和11.59%。然而,L-1细胞系中的内源精胺质量浓度虽有所提升,但与对照相比并无显著差异。当添加50 mg·L−1精胺时,L-1、L-2和L-3细胞系的内源精胺质量浓度分别达4 441.76、4 336.37和4 411.99 ng·L−1,与ck相比分别提高了16.11%,22.15%和19.02% (图4E)。
外源添加10 mg·L−1腐胺和20 mg·L−1亚精胺对3个细胞系的内源亚精胺质量浓度均无显著影响。外源添加50 mg·L−1精胺则显著提高了L-1、L-2和L-3细胞系的内源亚精胺质量浓度(P<0.05),与ck相比分别提高了31.69%、34.24%和31.01% (图4F)。
-
多胺通常被认为是一种植物体胚发生中的新型生长调节剂[19]。研究发现:外源多胺能提高橡胶树Hevea brasiliensis体细胞胚胎发生及成株的概率[20]。对荔枝Litchi chinensis的研究发现:在愈伤组织增殖培养基上添加腐胺、精胺和亚精胺可促进愈伤组织增殖并保持胚性[21]。本研究通过添加一定质量浓度的外源多胺能促进红松胚性细胞系的增殖率,并且不同基因型的细胞系对多胺种类和质量浓度的响应不同,其中10 mg·L−1 腐胺、50 mg·L−1精胺和20 mg·L−1 亚精胺对3个细胞系的增殖效果较好。
过氧化氢被认为是调节多种胁迫诱导反应的共同信号分子,可以通过丝裂原活化蛋白激酶(MAPK)级联调节等胁迫特异性反应[22]。氧化还原胁迫会提高胚性愈伤组织中的活性氧(ROS)水平,目前许多植物中的过氧化氢酶、超氧化物歧化酶抗氧化基因和酶系统已经得到了很好的表征[23−24]。有研究认为:胚性愈伤组织的再生能力与过氧化物酶活性的增加有密切联系[25],在白云杉Picea glauca研究中发现:胚性愈伤组织的过氧化物酶活性比非胚性愈伤组织低数倍,因此这些酶被认为是生化特性改变的重要指标[26]。胡忠等[27]研究认为:不同的多胺及其抑制剂可影响宁夏枸杞Lycium barbarum体胚中蛋白质和过氧化物同工酶的表达。本研究发现:与ck相比,外源添加10 mg·L−1腐胺、50 mg·L−1精胺和20 mg·L−1亚精胺,能很好地降低过氧化氢质量摩尔浓度以及过氧化物酶活性。因此,推测多胺能够参与到植物的活性氧代谢相关进程中,以促进红松胚性愈伤组织的增殖,这与水曲柳Fraxinus mandshurica[28]的研究结果相类似。
维持激素的动态平衡在诱导体胚胎发生中有重要作用,内源吲哚乙酸、脱落酸和乙烯水平的动态变化是调节体胚发生能力的关键信号[29−30]。在红松增殖阶段,胚性愈伤组织的优良生长为后期红松体胚的发育提供了更多机会。相关研究表明:柑橘Citrus reticulata愈伤中多胺的变化能够影响愈伤的体细胞胚胎发生能力[31]。本研究表明:外源添加50 mg·L−1精胺和20 mg·L−1亚精胺能显著提高内源吲哚乙酸质量浓度,有利于胚性愈伤组织的生长,外源添加50 mg·L−1精胺能显著提高内源脱落酸质量浓度。在南洋杉Araucaria cunninghamii的研究中,外源多胺的添加也促进了内源吲哚乙酸和脱落酸的积累[32]。这些结果表明:在红松胚性愈伤组织内多胺与植物激素有着密切联系,在多胺和乙烯合成途径中对相同底物S-腺苷甲硫氨酸(SAM)存在竞争[33]。本研究发现:外源施用10 mg·L−1腐胺和50 mg·L−1精胺能够促进红松L-1和L-2细胞系的内源乙烯积累。同时,外源多胺的添加对3个细胞系内源多胺质量浓度具有普遍的促进作用。因此,推测通过添加外源多胺促进了细胞内源多胺和乙烯的竞争底物SAM从前体中的释放,从而导致内源多胺和乙烯质量浓度都有所提高。此外,外源多胺对胚性愈伤组织的增殖和体细胞胚胎发生具有显著的促进作用。如PAUL等[34]研究表明:外源腐胺可使苦瓜Momordica charantia胚性愈伤组织鲜质量和体细胞胚胎发生率分别增加5.0和2.5倍。SATISH等[35]研究认为:外源亚精胺可有效促进小米Setaria italica胚性愈伤组织的增殖和体细胞胚胎的发生。本研究进一步证实:添加适当质量浓度的外源多胺可以提高红松胚性愈伤组织的内源多胺质量浓度,并在增殖过程中起着一定的促进作用。
-
外源多胺对红松胚性细胞增殖起到一定积极作用,5~30 mg·L−1腐胺、40~50 mg·L−1精胺和10~20 mg·L−1亚精胺对红松胚性愈伤组织的增殖促进效果较好。外源添加多胺,能够提高细胞内源多胺的水平、抗氧化能力和内源吲哚乙酸的水平,从而促进红松胚性愈伤组织的增殖。本研究结果有助于促进红松体细胞胚胎发生技术的改善,也为其他针叶树体胚发生技术的完善提供参考。
Effects of exogenous polyamines on proliferation of embryogenic calli of Pinus koraiensis
doi: 10.11833/j.issn.2095-0756.20250192
- Received Date: 2025-03-12
- Accepted Date: 2025-09-17
- Rev Recd Date: 2025-09-15
- Available Online: 2026-01-27
- Publish Date: 2026-02-20
-
Key words:
- Pinus koraiensis /
- embryogenic calli /
- polyamine /
- reactive oxygen species metabolism /
- plant hormones
Abstract:
| Citation: | LIU Shanshan, YANG Jianfei, SHEN Hailong. Effects of exogenous polyamines on proliferation of embryogenic calli of Pinus koraiensis[J]. Journal of Zhejiang A&F University, 2026, 43(1): 96−104 doi: 10.11833/j.issn.2095-0756.20250192 |









 DownLoad:
DownLoad: