-
天竺葵Pelargonium spp.为牻牛儿苗科Geraniaceae天竺葵属Pelargonium多年生草本植物或亚灌木,原产于非洲南部[1−2]。天竺葵属包含约280个种类,经长期人工栽培和选育形成了许多园艺品种,因其多季开花、花色艳丽、花叶兼美、适生性强且全株具有芳香等特性,已成为全球重要的观赏和芳香植物[3−4]。当前对天竺葵的研究多聚焦于扦插繁殖、抗病育种、组培快繁、精油开发等领域[5−9],而其生殖生物学的基础研究则相对匮乏。花粉活力水平直接决定杂交授粉的成功率,是种质创新与品种选育的关键前提[10]。花粉离体萌发法测得的花粉活力与实际萌发率最为接近[11]。矾根属Heuchera、鸢尾属Iris等植物[12−13]的研究表明:蔗糖、聚乙二醇4000 (PEG 4000)、硼酸等的合理组合可有效提升花粉离体萌发率。柱头是植物雌性生殖器官的核心部分之一,对芍药Paeonia lactiflora、兜兰属Paphiopedilum等研究[14−15]显示:柱头可授性是适配花粉识别、萌发及花粉管伸长的必要条件,决定了受精过程能否顺利完成。为探究天竺葵花粉活力及柱头可授性情况,本研究以无性系天竺葵品种为材料,研究不同培养基组分和质量浓度对花粉离体萌发的影响,并探明不同发育状态及环境条件下柱头可授性的变化规律,以期为天竺葵杂交育种的亲本选择与人工授粉时机的把握提供理论依据。
-
试验于2025年3—5月进行,供试品种为种植于玻璃连栋温室内,处于春季盛花期的不同天竺葵品种。选取天竺葵‘大草原 亮粉色’‘Savannah Hot Pink’花粉和柱头,分别作为花粉离体培养基筛选和柱头可授性试验的材料。选取9个天竺葵品种作为花粉活力测定的材料,分别是‘大草原 粉色飞溅’‘Savannah Pink Mega Splash’、‘大草原 酒红闪耀’‘Savannah TexMex Merlot Sizzle’、‘大力水手 粉色飞溅’‘Big EEZE Pink Splash’、‘大力水手 红色’‘Big EEZE Red’、‘大力水手 苹果花飞溅’‘Big EEZE Apple Blossom Splash’、‘桑塔纳 白色飞溅’‘Santana White Splash’、‘桑塔纳 紫色’‘Santana Purple’、‘天使之眼 糖果’‘Angel Eyes Candy’、‘优雅 宝诺娃’‘Elegance Bravo’。在晴天8:00—10:00,使用尖头镊子剥取盛开花朵内新鲜裂开的花药,将花粉收集至2 mL离心管中,置于冰箱4 ℃避光保存。本研究使用新鲜采集或4 ℃储藏不超过2 h的花粉。
-
参考矾根花粉离体萌发研究方法[12],本研究采用液体离体培养法,设置了蔗糖(A)、硼酸(B)、PEG 4000 (C)和氯化钙(D) 4个因素,每因素设置4个水平进行L16(44)正交设计试验(表1),每个处理重复3次。
水平 因素 蔗糖(A)/
(g·L−1)硼酸(B)/
(mg·L−1)PEG 4000(C)/
(g·L−1)氯化钙(D)/
(mg·L−1)1 0 0 0 0 2 50 50 100 5 3 100 150 200 10 4 150 250 300 15 Table 1. Experimental factors and levels
-
吸取适量液体培养基均匀滴加至凹面载玻片中,用尖头镊子取适量收集的花粉,均匀散布于培养基表面,并将凹面载玻片置于铺有湿润滤纸的培养皿内。将制备好的样本转移至人工气候培养箱中,培养箱温度为25 ℃,光照强度为8 klx,湿度为75%,培养4 h。在光学显微镜下进行观测,花粉管长度大于或等于花粉粒直径即视为萌发的花粉。每个处理3次重复,每次3个观察视野,每个视野需涵盖不少于50粒花粉。花粉萌发率=(萌发的花粉粒数/视野中花粉粒总数)×100%。
-
采用联苯胺-过氧化氢法检测柱头可授性。在盛花期的晴天10:00—12:00,用尖头镊子采不同发育状态的天竺葵柱头。同时,在盛花期的晴天和阴天8:00—16:00,每2 h用尖头镊子取平展状态的柱头,晴天和阴天5个采样时间点环境条件的平均值分别为:光照强度44.90和11.38 klx,温度27.70和22.32 ℃,湿度65.78%和87.06%。将采集到的柱头迅速浸入含有联苯胺-过氧化氢反应液[联苯胺(10 g·L−1)∶过氧化氢(30 g·L−1)∶水=4∶11∶22,体积比]的平面载玻片中,15 min后用尼康体视显微镜进行观察。若柱头周围的反应液有大量气泡出现,表明柱头具可授性;反之,则无。每个处理5次重复,并拍照。参考FERREIRA等[16]和焦雪辉等[17]的方法设置柱头可授性检验标准(表2)。
得分 气泡产生量 柱头可授性强度 0 无 无 1 极少 极弱(+) 2 较少 较强(++) 3 较多 强(+++) 4 大量 极强(++++) Table 2. Stigma receptivity test standard
-
采用正交设计助手ⅡV3.1分析并计算L16(44)正交设计中各因素试验平均值和极差值。使用Excel进行数据分析,采用SPSS 16.0进行单因素方差分析。
-
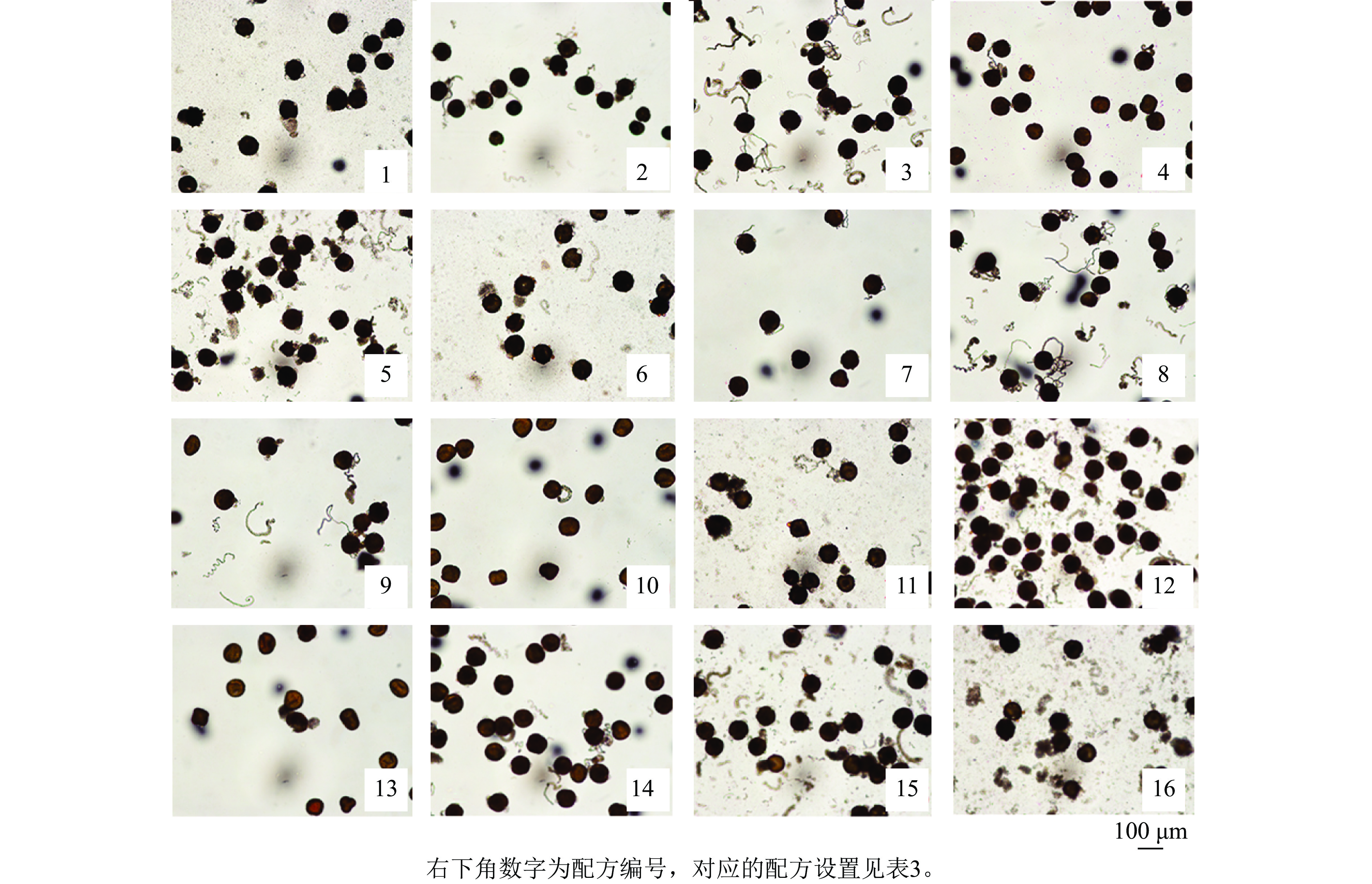
为探究不同因素对天竺葵花粉离体萌发的影响,观察了盛开期花粉在16种培养基配方上的萌发情况。结果显示:天竺葵花粉在不同培养基配方上的萌发率和花粉管伸长均存在明显差异(表3和图1),说明培养基配方对花粉萌发效果的影响十分明显。在培养基中各组分质量浓度均为0 (配方1,A1B1C1D1)的条件下,花粉萌发率为28.89%,花粉管较短;在配方8 (A2B4C3D2)条件下,花粉萌发率最高且显著高于其他处理(P<0.05),达68.89%,且花粉管伸长的速度较快,说明在合理添加供试4个组分的情况下,能够有效促进天竺葵花粉的离体萌发。而配方4 (A1B4C4D4)和配方10 (A3B2C4D3)的萌发率最低,仅为12.22%,且花粉管伸长的速度较慢(图1),说明过高质量浓度的组分添加反而会抑制天竺葵花粉的离体萌发。进一步分析各因素对萌发率的影响程度(表4),通过极差分析比较可知极差值(R)从大到小依次为:R(C)、R(B)、R(D)、R(A)。其中,PEG 4000的极差值最大,该因素下水平3均值(K3)是水平4均值(K4)的3.53倍,说明不同质量浓度的PEG 4000对花粉萌发效果的影响差异最大。蔗糖的极差最小,该因素下水平2均值(K2)是水平1均值 (K1)的1.24倍,说明蔗糖的影响最不明显。综合分析认为:PEG 4000是影响天竺葵花粉离体萌发最重要的组分,各组分的影响程度从高到低依次为PEG 4000、硼酸、氯化钙、蔗糖。筛选获得的天竺葵花粉离体萌发最佳培养基配方为50 g·L−1蔗糖+250 mg·L−1硼酸+200 g·L−1PEG 4000+5 mg·L−1氯化钙。
配方编号 配方设置 蔗糖/(g·L−1) 硼酸/(mg·L−1) PEG 4000/(g·L−1) 氯化钙/(mg·L−1) 花粉萌发率/% 1 A1B1C1D1 0 0 0 0 28.89±1.92 g 2 A1B2C2D2 0 50 100 5 48.89±5.09 de 3 A1B3C3D3 0 150 200 10 61.11±1.92 b 4 A1B4C4D4 0 250 300 15 12.22±1.92 h 5 A2B1C2D3 50 0 100 10 57.78±1.92 b 6 A2B2C1D4 50 50 0 15 37.78±3.85 f 7 A2B3C4D1 50 150 300 0 23.33±3.33 g 8 A2B4C3D2 50 250 200 5 68.89±3.85 a 9 A3B1C3D4 100 0 200 15 43.33±3.33 ef 10 A3B2C4D3 100 50 300 10 12.22±1.92 h 11 A3B3C1D2 100 150 0 5 56.67±3.33 bc 12 A3B4C2D1 100 250 100 0 61.11±5.09 b 13 A4B1C4D2 150 0 300 5 15.56±1.92 h 14 A4B2C3D1 150 50 200 0 50.00±3.33 cde 15 A4B3C2D4 150 150 100 15 54.44±1.92 bcd 16 A4B4C1D3 150 250 0 10 44.44±5.09 ef 说明:花粉萌发率数值为平均值±标准差,同列不同小写字母表示差异显著 (P<0.05)。 Table 3. Statistical results of pollen germination rate of ‘Savannah Hot Pink’ on different mediums
指标 花粉萌发率/% 蔗糖(A) 硼酸(B) PEG 4000(C) 氯化钙(D) K1 37.78 36.39 40.28 39.17 K2 46.94 37.22 55.56 47.50 K3 43.33 48.89 55.83 43.89 K4 41.11 46.67 15.83 36.94 R 9.17 12.50 40.00 10.56 说明:K1~K4分别表示各因素在水平1~4下花粉萌发率的平均值。R为极差值,即最大均值与最小均值之间的差值。 Table 4. Influence of different factors on pollen germination rate of ‘Savannah Hot Pink’
-
在最佳培养基配方的基础上,对不同品种的花粉活力进行测定。表5结果显示:天竺葵‘大力水手 粉色飞溅’的花粉萌发率最高,为76.67%,说明其花粉活力最高;其次为‘大草原 粉色飞溅’,萌发率为57.78%。‘大力水手 苹果花飞溅’的萌发率最低,为25.56%,说明其花粉活力最低。由此可见,不同品种间的花粉活力具有明显差异。
品种 花粉萌发率/% ‘大草原 粉色飞溅’ 57.78±5.09 ‘大草原 酒红闪耀’ 32.22±1.92 ‘大力水手 粉色飞溅’ 76.67±3.33 ‘大力水手 红色’ 55.56±5.09 ‘大力水手 苹果花飞溅’ 25.56±1.92 ‘桑塔纳 白色飞溅’ 28.89±1.92 ‘桑塔纳 紫色’ 38.89±3.85 ‘天使之眼 糖果’ 37.78±1.92 ‘优雅 宝诺娃’ 36.67±3.33 说明:数值为平均值±标准差。 Table 5. In vitro pollen germination rate of 9 cultivars
-
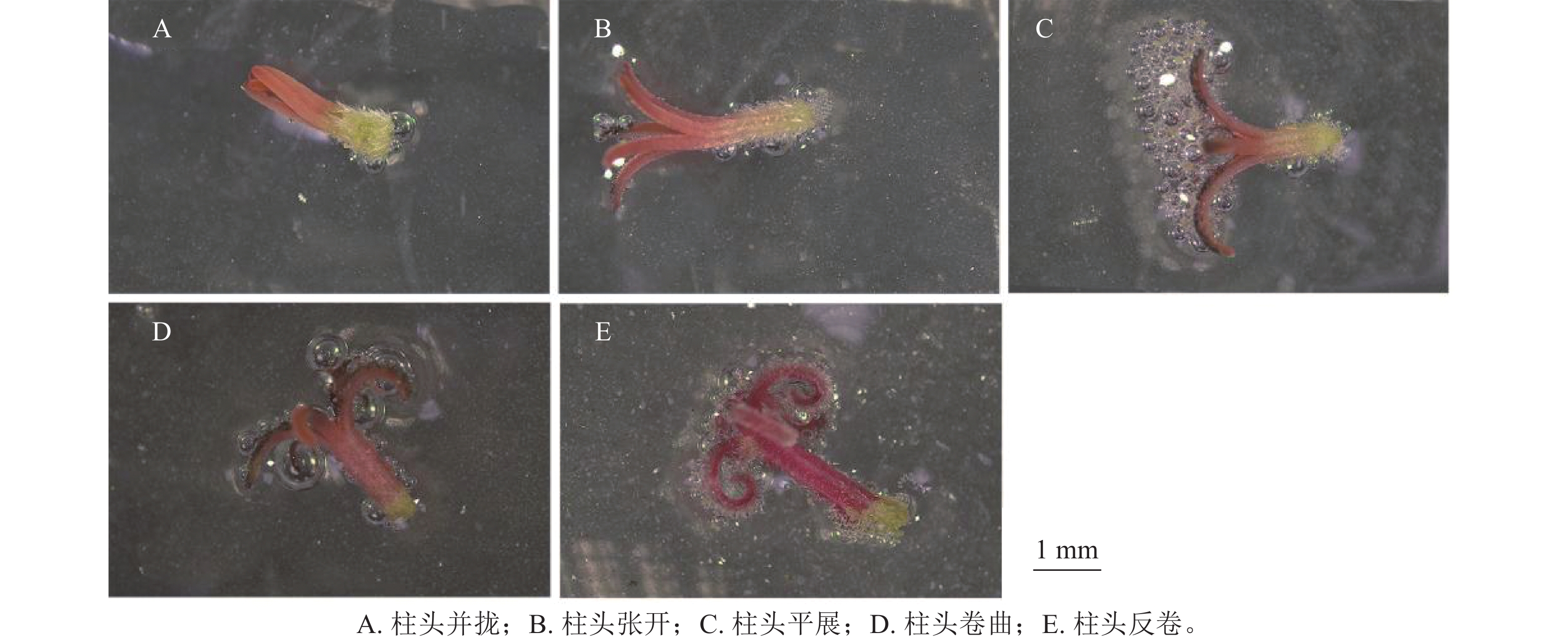
通过对不同发育状态下天竺葵‘大草原 亮粉色’柱头可授性情况及其分值(图2和表6)分析可知:并拢状态下的柱头几乎不产生气泡,柱头可授性分值显著低于其他状态(P<0.05),认为此状态下的柱头无可授性;在柱头张开状态下,气泡产生量极少,表明此时天竺葵柱头可授性极弱;与其他状态相比,平展状态的柱头有大量气泡产生,且柱头可授性分值显著高于其他状态(P<0.05),说明其可授性极强;而柱头在卷曲和反卷的状态下产生的气泡量均较少,说明柱头可授性相对较强(图2D~E)。随花朵发育进程,天竺葵柱头可授性会发生明显变化,呈现先增强再逐渐弱的趋势。据此认为在春季盛花期,呈平展状态的天竺葵柱头可授性最强,为授粉的最佳发育时期。
不同发育状态 柱头可授性分值 柱头并拢 0.60±0.55 d 柱头张开 1.60±0.55 c 柱头平展 3.80±0.45 a 柱头卷曲 2.40±0.55 b 柱头反卷 2.20±0.45 bc 说明:数值为平均值±标准差,同列不同小写字母表示差异显著(P<0.05)。 Table 6. Stigma receptivity of ‘Savannah Hot Pink’ under different developmental stages
-
在明确授粉最佳柱头发育时期的基础上,进一步研究柱头可授性的日变化规律。由表7可知:在盛花期8:00—16:00,天竺葵平展状态下的柱头均能检测到一定量的气泡,说明柱头均具有一定可授性。无论晴天还是阴天,检测到的气泡产生量都呈先上升后下降的趋势。当日柱头可授性分值的峰值均出现在10:00,且晴天10:00的分值显著高于其他时间(P<0.05),而最小的柱头可授性分值均出现在16:00,且阴天16:00的分值显著低于其他时间(P<0.05)。总体而言,天竺葵柱头晴天产生的气泡量多于阴天,上午多于下午,柱头可授性最大分值出现在晴天10:00,最小分值出现在阴天16:00。据此认为天竺葵盛花期柱头的可授性呈先升后降的日变化规律,最佳授粉时间为晴天的10:00左右。
时间 柱头可授性分值 晴天 阴天 8:00 2.60±0.55 b 2.80±0.45 a 10:00 3.80±0.58 a 3.20±0.58 a 12:00 2.80±0.00 b 2.60±0.58 a 14:00 1.60±0.00 c 1.80±0.00 b 16:00 1.40±0.58 c 0.80±0.00 c 说明:数值为平均值±标准差,同列不同小写字母表示差异显著(P<0.05)。 Table 7. Stigma receptivity of ‘Savannah Hot Pink’ at different time
-
PEG 4000是一种高分子渗透剂,能够起到保湿、维持细胞渗透压等作用,可通过改变花粉内膜的结构,调整膜表面的电荷分布,增强膜的柔韧性和通透性,进而有效促进花粉的萌发以及花粉管的生长[12, 17]。已有研究发现:PEG能够促进多种植物的花粉萌发,然而不同植物中的最适PEG 4000质量浓度有所不同,如铁甲秋海棠Begonia masoniana为50 g·L−1[18],10个菊花Chrysanthemum × morifolium品种为200 g·L−1[19],火麻Cannabis sativa为100 g·L−1[20],4种木兰属Magnolia植物为150~250 g·L−1[21]。在对不同类型蜡梅Chimonanthus praecox的研究中发现:PEG 4000是花粉离体培养所必需的培养基成分,最适质量浓度均为250 g·L−1[22−23]。对矾根花粉离体萌发的研究中认为PEG 4000不可或缺且起主导作用,最适质量浓度均为200 g·L−1[12]。本研究结果同样显示:PEG 4000是影响天竺葵花粉离体萌发最重要的组分,最佳质量浓度为200 g·L−1。因此推测,PEG 4000是天竺葵属植物花粉离体萌发培养基中最重要的组分,可考虑作为花粉离体培养基筛选和优化的首选因素开展相关试验,也可以为其他观赏植物花粉离体萌发培养基的筛选提供重要参考。
蔗糖作为花粉能量代谢与跨膜运输的核心供能物质,同步承担渗透调节功能以维持胞内稳态[13]。研究表明:花粉离体培养基中的蔗糖质量浓度存在阈值效应,超阈值时引发的渗透胁迫反而抑制萌发[12−13, 21]。对蜡梅[22]和梅花Prunus mume[24]的研究均表明超阈值后花粉萌发率与质量浓度呈负相关。在本研究供试的4种因素中,尽管蔗糖对天竺葵花粉萌发的促进作用相对最小,但在设置的质量浓度范围内(0~150 g·L−1)花粉萌发率同样呈先升后降的趋势。因而,在选择蔗糖作为培养基组分以期达到促进花粉离体萌发效果时,应充分考虑其阈值效应。
锦带Weigela florida花粉离体萌发试验表明:培养基中硝酸钙对花粉萌发率的影响最为显著[25]。然而更多研究表明:钙离子并非花粉萌发的主导因子,而是发挥不可或缺的协同调控作用,其动态平衡对启动萌发及后续花粉管伸长均至关重要[26−27]。本研究结果显示:钙离子对天竺葵花粉萌发的影响效果仅高于蔗糖,适宜质量浓度(5~10 mg·L−1)氯化钙的添加能够促进萌发,过高质量浓度(15 mg·L−1)也会对萌发产生不利影响。说明钙离子并非影响天竺葵花粉离体萌发的主导因素,它与其他组分协同发挥作用。
天竺葵园艺品种数量丰富,类型多样,品种群是由属内不同种或品种之间经反复杂交而形成的[5, 8]。本研究供试的9个天竺葵品种大部分为市场化程度较高的无性系品种,它们之间的花粉活力存在明显差异。说明在长期人工栽培和选育过程中,随着无性系天竺葵品种占比的增加,品种间花粉活力状况产生了一定分化。因此,在育种亲本选择时应对花粉活力状况提前进行测定。推测这一现象与品种的遗传背景、花粉结构和发育状况等有关[28]。
-
柱头可授性是影响杂交亲本选择和杂交成功率的关键因素之一,柱头形态特征与受精窗口期密切相关,可作为人工授粉的重要参考[29−31]。宋一岚等[15]对兜兰‘绿肉饼’Paphiopedilum ‘Pacific Shamrock’柱头可授性的研究发现:其柱头可授性随开花时间先弱后强再变弱,开花10~20 d的柱头可授性最高。黄薇Heimia myrtifolia柱头在开花后48 h会彻底萎蔫,失去可授性[32]。本研究同样发现:天竺葵花朵开放后,柱头可授性呈现先增强再减弱的趋势,呈平展状态的柱头可授性最强。LI等[33]发现金银花Lonicera japonica在柱头开裂期可授性最强,与本研究中天竺葵在柱头平展状态下可授性最强的结果一致。已有研究表明:光照和气温等环境因素会对紫斑牡丹Paeonia rockii柱头可授性造成一定影响[34]。本研究发现在晴天10:00左右,平展状态的天竺葵柱头可授性最强,可能由于此时光照强度、温度和湿度最为适宜,促使柱头分泌大量黏液。柱头可授性从14:00开始明显下降,16:00为最低。因此,应该选择在晴天10:00左右,对平展状态的柱头进行授粉,能够在一定程度上提高授粉成功率。
-
本研究在优化花粉离体萌发最佳培养基配方的基础上建立了天竺葵花粉活力测定方法,获得不同品种花粉活力范围为25.56%~76.67%,建议优先选择花粉活力50%以上的品种作为杂交中的父本;并明确了柱头授粉的最佳发育时期和时间,有助于提升天竺葵杂交育种的效率。
Research on pollen viability determination and stigma receptivity of Pelargonium spp.
doi: 10.11833/j.issn.2095-0756.20250464
- Received Date: 2025-08-29
- Accepted Date: 2025-10-11
- Rev Recd Date: 2025-10-11
- Available Online: 2025-10-29
- Publish Date: 2025-10-20
-
Key words:
- Pelargonium /
- pollen viability /
- in vitro germination /
- stigma receptivity /
- orthogonal design
Abstract:
| Citation: | WANG Wenjing, XU Junxu, WANG Hongbing, et al. Research on pollen viability determination and stigma receptivity of Pelargonium spp.[J]. Journal of Zhejiang A&F University, 2025, 42(5): 975−983 doi: 10.11833/j.issn.2095-0756.20250464 |









 DownLoad:
DownLoad: