-
果胶类多糖广泛分布于高等植物的根、茎、叶、果实等的细胞初生壁和细胞间隙,对细胞组织起着软化和黏合作用,同时还是抵御病原体微生物入侵的天然屏障[1]。2-酮-3-脱氧辛糖酸(KDO)是果胶类多糖鼠李半乳糖醛酸聚糖-Ⅱ(Rhamnogalacturonans Ⅱ,RG-Ⅱ)成分中很少见的八碳糖[2],在进化过程中非常保守,对花粉管的伸长和生长具有一定的作用[3-4]。KDO合成过程中涉及到5种酶[5],其中阿拉伯糖-5-磷酸异构酶(D-arabinose 5-phosphate isomerase,KdsD)是KDO生物合成过程中的第1个关键酶,可以催化核酮糖-5-磷酸(D-ribulose 5-phosphate,Ru5P)的异构化,以产生在KDO生物合成途径中的第1个中间产物D-阿拉伯糖-5-磷酸(D-arabinose 5-phosphate,A5P)。KDO最初是在革兰氏阴性菌中发现的,它连接O-特异侧链与类脂A共同嵌入细胞壁外膜上[6]。细胞壁中KDO合成的阻断会导致类脂A物质的累积和细胞生长停滞[7]。研究发现,KDO生物合成途径也存在于藻类植物与高等植物中[2, 8]。大肠埃希菌Escherichia coli中的KdsD晶体结构已经被解析,它是一个同源四聚体[9-11]。每个KdsD亚基包含2个不同的结构域:N-末端糖异构酶(SIS)结构域,主要参与磷糖异构化作用,以及1对未知功能的胱硫醚-β-合酶(CBS)结构域。通过定点突变确定了大肠埃希菌的Lys59,His88和His193对应于绿脓杆菌Pseudomonas aeruginosa的Lys56,His85和His190[12-14]分别是其活性重要的保守残基。目前,植物中KdsD的研究很少,其晶体结构也尚不清楚,植物来源的KdsD酶功能及催化作用机制、酶的催化特性等科学问题仍需要更加深入研究。毛竹Phyllostachys edulis是中国广泛分布的一种经济价值很高的植物,在林业生产中的地位至关重要。本研究拟克隆毛竹KdsD基因,分析它在不同组织的表达特异性,进行生物信息学分析和原核表达,经过Ni-NTA亲和层析和分子筛层析(SEC)纯化获得高纯度的蛋白,并对其酶学性质进行分析,为后续KdsD晶体学结构和植物KdsD基因功能的研究打下理论基础。
-
毛竹采自浙江农林大学亚热带森林培育国家重点实验室培育基地,取半年生毛竹实生苗新鲜的根、茎、叶分装于冻存管中,于液氮中速冻,放-80 ℃冰箱备用。
-
取毛竹新鲜的根、茎、叶在液氮中迅速研磨成粉末,用Trizol法[15]提取总RNA,用脱氧核糖核酸酶Ⅰ(DNaseⅠ)消除DNA污染。分别以毛竹约1.0 μg的根、茎、叶RNA为模板,利用逆转录试剂盒合成cDNA。
-
通过美国生物技术信息中心(NCBI)数据库获得拟南芥Arabidopsis thaliana的KdsD基因序列,检索毛竹数据库,得到一条同源序列。根据该基因的开放读码框(ORF)设计引物。上游BamH I酶切位点引物5′-CGGGATCCATGGGCTCGCTCCCCGTGCCCT-3′;下游Hind Ⅲ酶切位点引物5′-CCAAGCTTTTATAATCCAGCAGAGACCAAT-3′(酶切位点用下划线显示)。引物由上海生工生物工程股份有限公司合成。以反转录后的cDNA 为模板进行聚合酶链式反应(PCR)扩增。将PCR扩增产物电泳分析后经DNA胶回收试剂盒回收,获得的目的DNA片段插入经同样酶切后的pE-SUMO表达载体,得到1个N-末端含有6个His亲和标签的重组质粒。将重组质粒转化到大肠杆菌DH5α中,提取阳性克隆质粒进行双酶切和单酶切验证。
-
根据毛竹KdsD基因的保守序列设计qRT-PCR引物(正向引物:GTGTTGGATTCTGGATGGTTC;反向引物:CAGCCGTAGTGGTGAATGAGTAAC)。内参Actin(正向引物:CGACATTGGCTCGCTCTTC;反向引物:CGGCAGAGGTGAGGGAGAT),使用荧光定量PCR仪对PeKdsD基因在根、茎、叶不同组织的表达量进行分析。
-
将构建好的重组表达质粒转化到表达菌株E. coli(Ril)中,挑取单克隆,接入2 mL 溶菌肉汤(LB)培养基(A+)37 ℃培养,当菌体吸光度D(600)达到0.5~0.8时,加入终浓度为0.4 mmol·L-1的异丙基硫代半乳糖苷(IPTG)诱导,分别在37 ℃摇床上诱导3 h,在20 ℃摇床上过夜诱导,最后收集菌体,超声破碎,用十二烷基硫酸钠-聚丙烯酰氨凝胶电泳(SDS-PAGE)检测蛋白表达情况。
-
在确定的最佳温度表达条件下,将菌株接种于1.0 L LB培养基中进行扩大培养。加入诱导剂后,菌液在20 ℃,220 r·min-1条件下过夜培养。离心收集菌体,将裂解液[1.0 mol·L-1氯化钠,30.0 mmol·L-1三羟甲基氨基甲烷(Tris-HCl),体积分数为5%的甘油]加入到收集的菌体中,超声破碎细胞后,20 000 r·min-1,4 ℃条件下离心45 min,将上清转移至镍柱,置于摇床缓慢结合1 h,后分别用低浓度咪唑缓冲液洗脱杂蛋白,用高浓度咪唑缓冲液洗脱目的蛋白。将目的蛋白收集在10 kDa浓缩柱中离心进行浓缩,至体积为500.0 μL左右。使用分子筛层析柱SuperoseTM 12 10/300 GL上样,根据蛋白的出峰顺序收集蛋白,用SDS-PAGE胶检测蛋白的纯度,将较高纯度的蛋白收集浓缩,置于-80 ℃保存。
-
酶活力测定采用半胱氨酸-咔唑法[16-17]。25.0 μL的酶用100.0 mmol·L-1 Tris-HCl,pH 8.5配制,37 ℃保温3 min,加入25.0 μL 2.0 mmol·L-1 阿拉伯糖-5-磷酸(A5P),37 ℃反应5 min,加入50.0 μL 12.5 mol·L-1硫酸终止反应,采用半胱氨酸-咔唑法显色,测定D(540) nm吸光度。酶活力单位(1 U=16.67 nkat)定义为: 以A5P为底物,每分钟催化产生1.0 μmol的核酮糖-5-磷酸(Ru5P)所需的酶量。所有酶活测定结果均为3次重复平行试验数据的平均值。
-
最适pH值的测定,将样品KdsD稀释在100.0 mmol·L-1不同pH值缓冲液中,MES缓冲液从pH 6.0~6.5,Tris-HCl缓冲液从pH 7.0~9.0。37 ℃反应5 min,测定酶活。最适温度则是在测定的最适pH值下,将反应温度控制在25~65 ℃,隔10 ℃进行酶促反应,然后测定酶活性。
-
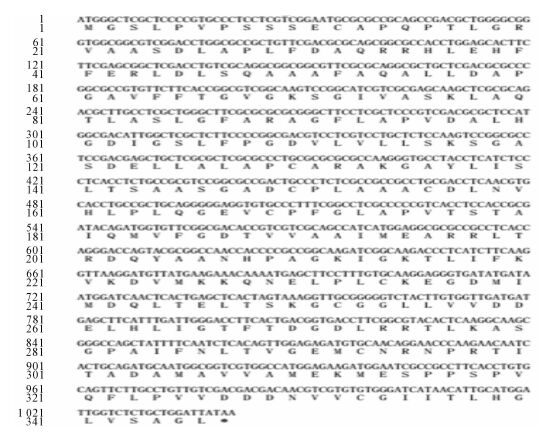
PeKdsD基因经PCR扩增重组后测序,得到1条开放读码框(ORF)为1 038 bp的目的片段。该基因编码346个氨基酸(图 1)。将PCR产物与pE-SUMO表达载体连接,产物转化到DH5α,挑取阳性菌落进行菌检测出,成功构建原核表达载体。
-
通过blast在线软件分析,结果显示,PeKdsD含有2个不同的结构域:SIS结构域和CBS结构域,大肠埃希菌序列中关键的催化残基Lys59,His88和His193在毛竹中是完全保守的。PeKdsD编码的氨基酸序列与玉米Zea mays,高粱Sorghum bicolor和水稻Oryza sativa具有较高的一致性,均为91.00%,与杨树Populus trichocarpa和拟南芥的一致性分别为69.00%和66.38%,而与大肠埃希菌和脆弱拟杆菌Bacteroides fragilis的一致性分别只有29.21%和31.12%(图 2)。采用MEGA6软件构建不同物种KdsD氨基酸序列的系统进化树(图 3),结果表明:毛竹与玉米位置较近,位于同一个分支上;与杨树、水稻位于同一大分支上,与拟南芥和脆弱拟杆菌分支较远。
-
PeKdsD不同组织qRT-PCR结果显示:PeKdsD在毛竹的根、茎、叶中均有表达,但在表达量上存在较大的差异,在叶中的表达量远远大于根与茎(图 4)。
-
将PeKdsD重组载体转化到表达菌株E. coli(Ril)感受态细胞,使用IPTG诱导表达,SDS-PAGE检测蛋白表达及可溶性情况(图 5A)。对比发现表达菌株Ril在20 ℃培养温度下表达量相对较高,故在后续试验中确定重组蛋白诱导表达的最佳温度为20 ℃。
-
将收集的菌体超声破碎离心后,上清加入Ni-NTA柱,冰上结合后,采用咪唑梯度洗脱蛋白,SDS-PAGE检测(图 5B)。结果显示:条带出现的位置与目的蛋白大小一致,目的蛋白被成功地纯化出来。将目的蛋白进一步经SuperoseTM 12 10/300 GL分子筛层析后,出现2个明显的吸收峰。对比小牛血清BSA出峰位置(二聚体C峰对应分子量为132.86 kDa,单聚体D峰对应分子为66.43 kDa),A峰对应是KdsD多聚体,B峰对应的是KdsD单体(图 6),说明KdsD蛋白在溶液中存在多种聚体形式。2步纯化Ni-NTA结合SEC可分离纯化得到不同状态的高纯度KdsD蛋白,可满足今后酶学特性的研究和蛋白晶体生长的要求。
-
酶活性测定结果表明:纯化的KdsD酶最适温度为37 ℃,在35~45 ℃范围内酶活力较高,45 ℃以上酶活力急剧下降,而在25 ℃时仍保持在60%以上(图 7A);不同的pH值条件也会影响酶的活性,最适作用pH值为pH 8.5,此时活性较高,过酸或过碱环境都会对酶活性有较大的抑制作用(图 7B)。
-
自2010年大肠埃希菌的KdsD结构公布后,又陆续有其他微生物的KdsD结构报道。2014年CHIU等[14]解析出了脆弱拟杆菌API(bfAPI)的晶体结构,为四聚体。但是植物来源的KdsD相关研究甚少,其结构和功能至今仍未被解析。本研究首次成功克隆到了毛竹KdsD基因,该基因具有一个完整的开放阅读框(ORF),编码346个氨基酸。生物信息学分析预测其含有2个不同的结构域:N-末端糖异构酶(SIS)结构域和胱硫醚-β-合酶(CBS)结构域。尽管大肠埃希菌序列中关键的催化残基Lys59,His88和His193在毛竹中是完全保守的,但是毛竹KdsD蛋白序列与大肠埃希菌KdsD蛋白序列相似度不足30%,因此,推测植物KdsD与微生物中的KdsD蛋白空间结构和功能可能存在一定的差异。为进一步了解植物KdsD和微生物KdsD结构上的差异,还需进一步纯化获得高纯度的蛋白,进行蛋白结晶,通过X射线等方面的研究进一步分析验证结构与功能的关系。实时定量PCR在根、茎、叶中均检测到有表达,尤其在叶中表达量比其他组织高约5~8倍,表现出明显的组织特异性现象。Ni-NTA纯化显示所得的目的蛋白大小与预测的蛋白分子量相一致,成功纯化出了PeKdsD蛋白。进一步的SEC纯化结果表明:毛竹KdsD在30 mmol·L-1 Tris-HCI pH 8.0,200 mmol·L-1氯化钠溶液条件,以多聚体混合的形式存在,SEC中KdsD的多聚体究竟是几聚体还需分析超速离心(AUC)等实验做进一步的分析验证。酶学性质初步测定结果表明:PeKdsD的最适温度为37 ℃,最适pH值为pH 8.5。最适pH值与预测的pH值有所偏差,故后期试验采用pH 8.5为宜。当然,要揭示毛竹KdsD酶功能及催化作用机制,还有待于后续晶体结构与功能等方面的进一步深入研究。
Gene cloning, expression, and purification of the arabinose-5-phosphate isomerase from Phyllostachys edulis
-
摘要: 毛竹Phyllostachys edulis阿拉伯糖-5-磷酸异构酶(PeKdsD)是2-酮-3-脱氧辛糖酸(KDO)生物合成途径的第1个关键酶。采用逆转录实时聚合酶链式反应(qRT-PCR)克隆得到毛竹KdsD基因。该基因cDNA全长1 038 bp,编码346个氨基酸;对不同物种来源的KdsD氨基酸序列进行比对和系统进化树分析。结果表明:毛竹的KdsD与玉米Zea mays等植物来源的KdsD有很高的序列一致性,而与微生物来源的KdsD序列一致性较低。毛竹不同组织实时荧光定量聚合酶链式反应(qRT-PCR)结果表明:PeKdsD基因在叶中表达量远远高于其他组织。在大肠埃希菌Escherichia coli原核表达系统中获得了KdsD的可溶性高表达蛋白,将大量表达的重组蛋白经过Ni-NTA亲和层析和分子筛层析(SEC)进行纯化,发现KdsD在溶液(30 mmol·L-1三羟甲基氨基甲烷pH 8.0,200 mmol·L-1氯化钠)中以多种聚体的形式存在;酶学性质测定的结果表明:毛竹KdsD酶最适pH值为pH 8.5,最适作用温度为37℃。此结果为后期研究植物KdsD蛋白的结构与功能奠定了基础。图7参17Abstract: Phyllostachys edulis arabinose-5-phosphate isomerase (PeKdsD) is the first key enzyme in the biosynthesis of 3-deoxy-D-manno-octulosonate (KDO). The full length cDNA of the KdsD gene (1 038 bp with 346 amino acids) was cloned using reverse transcription polymerase chain reaction (qRT-PCR) followed by protein Basic Local Alignment Search Tool (BLAST) and phylogenetic tree analysis of the amino acid sequence. Then a qRT-PCR analysis of diverse tissues in Ph. edulis was conducted, and a mass of soluble KdsD protein was obtained through a prokaryotic expression system in Escherichia coli. The recombinant protein was further purified through Ni-NTA affinity and size exclusion chromatography (SEC) in a buffer of 30 mmol·L-1 Tris-HCl pH 8.5, 200 mmol·L-1 NaCl to determine enzymatic properties. Results of the amino acid sequence among different species indicated that the PeKdsD isomerase had a high sequence similarity with KdsD in Zea mays but was low in microorganisms. The qRT-PCR analysis of diverse tissues in Ph. edulis revealed that PeKdsD had its highest expression level in the leaf. SEC results showed that the recombinant KdsD protein existed as protein polymers, and for enzymatic properties of PeKdsD the optimum reaction temperature was 37℃ with a pH of 8.5. This work provides a basis for determining the structure and function of plant KdsD.[Ch, 7 fig. 17 ref.]
-
-
[1] 孙元琳,汤坚. 果胶类多糖的研究进展[J]. 食品与机械, 2004, 20(6):60-63. SUN Yuanlin, TANG Jian. Research progress in petic polysaccharides[J]. Food Mach, 2004, 20(6):60-63. [2] YORK W S, DARVILL A G, MCNEIL M, et al. 3-deoxy-d-manno-2-octulosonic acid (KDO) is a component of rhamnogalacturonan Ⅱ, a pectic polysaccharide in the primary cell walls of plants[J]. Carbohydr Res, 1985, 138(1):109-126. [3] SÉVENO M, SÉVENO-CARPENTIER E, VOXEUR A, et al. Characterization of a putative 3-deoxy-D-manno-2-octulosonic acid (Kdo) transferase gene from Arabidopsis thaliana[J]. Glycobiology, 2010, 20(5):617-628. [4] LI Yi. Studies of 3-Deoxy-D-manno-Octulosonate 8-Phosphate Phosphatase:Mechanistic Insights and a Gene Fusion Example[M]. Ann Arbor:ProQuest, 2009. [5] DELMAS F, SÉVENO M, NORTHEY J G B, et al. The synthesis of the rhamnogalacturonan(Ⅱ)component 3-deoxy-D-manno-2-octulosonic acid (Kdo) is required for pollen tube growth and elongation[J]. J Exper Bot, 2008, 59(10):2639-2647. [6] SMYTH K M, MARCHANT A. Conservation of the 2-keto-3-deoxymanno-octulosonic acid (Kdo) biosynthesis pathway between plants and bacteria[J]. Carboydr Res, 2013, 380(2):70-75. [7] RAETZ C R. Biochemistry of endotoxins[J]. Ann Rev Biochem, 1990, 59(1):129-170. [8] BECKER B, LOMMERSE J P M, MELKONIAN M, et al. The structure of an acidic trisaccharide component from a cell wall polysaccharide preparation of the green alga Tetraselmis striata Butcher[J]. Carbohydr Res, 1995, 267(2):313-321. [9] MEREDITH T C, WOODARD R W. Identification of GutQ from Escherichia coli as a D-arabinose 5-phosphate isomerase[J]. J Bacteriol, 2005, 187(20):6936-6942. [10] SPERANDEO P, POZZI C, DEHÒG, et al. Non-essential KDO biosynthesis and new essential cell envelope biogenesis genes in the Escherichia coli yrbG-yhbG locus[J]. Res Microbiol, 2006, 157(6):547-558. [11] MEREDITH T C, WOODARD R W. Escherichia coli YrbH is a D-arabinose 5-phosphate isomerase[J]. J Biol Chem, 2003, 278(35):32771-32777. [12] SOMMARUGA S, de GIOIA L, TORTORA P, et al. Structure prediction and functional analysis of KdsD, an enzyme involved in lipopolysaccharide biosynthesis[J]. Biochem Biophysi Res Commun, 2009, 388(2):222-227. [13] AIROLDI C, SOMMARUGA S, MERLO S, et al. Targeting bacterial membranes:identification of Pseudomonas aeruginosa D-Arabinose-5P isomerase and NMR characterisation of its substrate recognition and binding properties[J]. Chem BioChem, 2011, 12(5):719-727. [14] CHIU H J, GRANT J C, FARR C L, et al. Structural analysis of arabinose-5-phosphate isomerase from Bacteroides fragilis and functional implications[J]. Acta Crystallogr, 2014, 70(10):2640-2651. [15] 吴凯朝, 黄诚梅, 李杨瑞, 等. Trizol试剂法快速高效提取3种作物不同组织总RNA[J]. 南方农业学报, 2012, 12(12):1934-1939. WU Kaichao, HUANG Chengmei, LI Yangrui, et al. Fast and effective total RNA extraction from different tissues in 3 crops through the Trizol reagent method[J]. J Southern Agric, 2012, 12(12):1934-1939. [16] DISCHE Z, BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses[J]. J Biol Chem, 1951, 192(2):583-587. [17] MOSBERG J A, YEP A, MEREDITH T C, et al. A unique arabinose 5-phosphate isomerase found within a genomic island associated with the uropathogenicity of Escherichia coli CFT073[J]. J Bacteriol, 2011, 193(12):2981-2988. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.2016.06.002







 下载:
下载: