-
植物进行光合作用的主要器官是叶片,研究发现不仅植物叶片能够进行光合作用,其他绿色非叶器官也能够进行光合作用[1-3]。NILSEN[3]发现植物绿色茎秆具有与叶片相似的光合能力;MANETAS[4]研究发现大量的光照(10%~50%)是被树干吸收的;SUN等[5]研究表明:树干木质部和韧皮部纤维以及管胞管壁对不同方向照射光线具有良好的茎向导光特性。HIBBERD等[6]发现:C3植物烟草Nicotiana tabacum和芹菜Apium graveliens茎中具有C4光合途径。NILSEN[3]认为虽然景天酸代谢途径(CAM)植物叶片多为C3途径,但其茎秆多为CAM途径,其他植物茎秆光合为C3途径。占东霞等[7]对棉花Gossypium hirsutum叶片和非同化器官的研究证明了非同化器官对光合作用的贡献。类囊体是叶绿体中光能向化学能转化的主要场所,一直是光合作用研究的热点[8]。类囊体膜又称光合膜,光合作用的4个多亚基蛋白复合体——光系统Ⅱ(PSⅡ)、光系统Ⅰ(PSⅠ)、ATP合成酶(ATP-ase)和细胞色素b6f复合体(Cytb6f)都定位在类囊体膜上[9-10],这些蛋白复合体和许多其他辅助因子共同完成光合电子传递和光合磷酸化的过程[11]。高荣孚等[12]用含有聚乙二醇(PEG)的提取液提取和分离了油松Pinus tabulaeformis和豌豆Pisum sativum的类囊体膜,并得到完整的PSⅠ,证明存在2种PSⅠ,而且这种存在具有一定普遍性。蔡霞等[13]在低温(83 K)下利用稳态荧光光谱技术对PSⅡ核心复合物中激发能的传递进行了研究,发现最大峰所在位置没有因激发波长的不同而发生改变;在不同波长光的激发下,核心复合物中能量传递的途径不同。周伟等[14]利用蓝绿温和胶电泳(BN-PAGE)、结合质谱鉴定对条斑紫菜Porphyra yezoensis类囊体膜蛋白复合物进行了研究,检测到PSⅡ核心复合物中D2、D1、CP47、CP43和Cytochrome f等蛋白。在分子水平上揭示各种膜蛋白复合体的结构与功能,对于揭示光能转化的机理具有重要意义[15]。毛竹Phyllostachys edulis是江浙地区极富经济和生态价值的竹种。目前,对毛竹的研究主要在毛竹光合生理特性[16]、茎叶绿素荧光特征和光合酶活性[17-18]、碳水化合物代谢[19-20]、蛋白组学[21]和基因组学[22]等方面。关于毛竹茎秆类囊体膜蛋白复合物、快速生长期毛竹茎秆光合特性的研究未见报道。本研究通过分析毛竹茎秆快速生长期叶片和茎秆的光合色素含量、77 K低温荧光发射光谱、蓝绿温和胶电泳来揭示类囊体膜蛋白复合物的变化,为阐明毛竹茎秆光合作用机理提供理论依据。
-
2018年5月初,在浙江省杭州市临安区现代毛竹示范园内,选取生境条件一致、生长状况良好、株高(6.0±0.2) m、基径约15 cm、自然状态下的当年生毛竹笋竹,从茎秆基部将其伐倒,将节间按照从基部至顶部的顺序编号,1~6节(笋衣完全脱落)为茎秆基部,7~13节(笋衣开始脱落,茎秆呈现绿色)为茎秆中部,13节以上(笋衣包裹完好,茎秆为黄色)为茎秆顶部,取茎秆外层表皮,厚度为<3 mm。6月初,选择枝条梢部下3~4位无病斑的当年生叶片,取样时间为10: 00−12: 00。取样后直接进行发射荧光的测定;用于光合色素和电泳样品取下后,迅速将样品放进液氮中冷冻,存于−80 ℃备用。
-
采用ARNON[23]的方法略做修改。称取毛竹叶片和茎秆0.5 g,剪碎后置于带盖的试管中,加体积分数为80%丙酮溶液5 mL,在暗处浸提48 h,分别在470、645和663 nm处测定其光密度D(λ),参考LICHTENTHALER[24]的方法,计算叶绿素a(Chl a)、叶绿素b(Chl b)和类胡萝卜素(Car)质量分数。选取5株笋竹和5株成竹,每株作为1个独立实验,共5次重复。
-
利用AvaSpec-HS-TEC超高灵敏型光纤光谱仪(北京爱万提斯科技有限公司),在液氮(77 K)中测量活体状态下毛竹叶片和茎秆荧光发射光谱。采用陈登举等[25]方法稍作修改,以AvaLight-LED为激发光源(480 nm),光照强度控制在3 000 μmol·m−2·s−1,发射波长范围为600~900 nm,发射步长为1 nm,扫描速度为500 nm·s−1。测量前先用标准白板校对调0,每个样品选择无病斑毛竹叶片和各部位茎秆材料各20片,每片采集数据1次。
-
采用SCHÄGGER等[26]方法稍作修改。称取毛竹叶片10 g和笋竹茎秆50 g,加入100 mL预冷的提取液(0.1 mol·L−1蔗糖,0.2 mol·L−1氯化钠,50 mmol·L−1 pH 7.4磷酸缓冲液,质量浓度为2.5%聚乙二醇6000)中,用50 g多功能粉碎机(上海市蒲恒信息科技有限公司)粉碎至匀浆中无明显碎片为止(大约1 min),将匀浆通过4层过滤,滤液3 000×g离心10 min (4 ℃),收集沉淀。将沉淀用10倍体积预冷后的清洗液(不含聚乙二醇的提取液)悬浮起来,3 000×g离心10 min (4 ℃),收集沉淀。再用5倍体积的冷清洗液将沉淀悬浮起来,500×g离心5 min,弃沉淀,将上层悬液3 000×g离心10 min (4 ℃),收集沉淀,并用悬浮液(0.3 mol·L−1蔗糖,50 mmol·L−1氯化钠,50 mmol·L−1磷酸缓冲液,pH 6.9)悬浮。得到的悬液即为类囊体膜蛋白制备液,测定叶绿素质量分数后储藏于−20 ℃冰箱中。
-
电泳样品制备:取类囊体膜蛋白制备液1 000 μL (约含10 000 μg蛋白和1 000 μg叶绿素),3 000×g离心10 min(4 ℃),取沉淀,用1 000 μL上样缓冲液[ACA缓冲液:750 mmol·L−1氨基己酸,50 mmol·L−1 pH 7.0双(2-羟乙基)氨基(三羟甲基)甲烷(Bis-Tris),0.5 mmol·L−1乙二胺四乙酸(EDTA)]悬浮,再加入0.05 g毛地黄皂苷,混匀使之完全溶解,在冰浴中用JY88-IIN超声波细胞粉碎机(宁波新芝生物科技股份有限公司)处理混合液1 min,4 ℃下放置30 min,然后8 780×g离心30 min,取上清液,加入5 μL考马斯亮蓝染液(质量浓度为5%考马斯亮蓝G250,750 mmol·L−1氨基己酸),混匀后上样。第1向电泳:胶体由质量浓度为4%浓缩胶和5%~13%梯度分离胶组成,上样量为30 μL。加入阳极缓冲液(50 mmol·L−1Bis-Tris-HCl,pH 7.0)和阴极缓冲液A [15 mmol·L−1 Bis-Tris,50 mmol·L−1 N-三(羟甲基)甲基甘氨酸(Tricine),质量浓度为0.02%考马斯亮蓝G250],80 V恒压电泳,当样品完全进入浓缩胶后,吸出阴极缓冲液A,换阴极缓冲液B (15 mmol·L−1 Bis-Tris,50 mmol·L−1 Tricine),120 V恒压直到电泳完成。整个电泳过程在4 ℃下完成。第2向电泳:将经蓝绿温和胶分离的类囊体膜蛋白复合物胶条切下,在室温下经样品处理液(质量分数为5%十二烷基硫酸钠(SDS),体积分数为20%甘油,体积分数为10%巯基乙醇,50 mmol·L−1 Tris-HCl,pH 6.8)处理30 min后,置于50 ℃水浴锅中加热5 min,然后用去离子水洗去巯基乙醇。把胶条放置在准备好的第2向胶体上,进行第2向电泳。电泳在室温条件下进行,25 mA恒流,阴极缓冲液:100 mmol·L−1 Tris-HCl,100 mmol·L−1 Tricine,质量浓度为0.1%SDS,pH 8.25,阳极缓冲液:100 mmol·L−1 Tris-HCl,pH 8.9。
-
荧光光谱采集得到的数据经Multispec 5.1初步处理,用Origin 9统计软件进行统计分析,经卷积平滑消除噪音后,经归一处理,采用高斯函数对荧光发射光谱进行拟合,肩峰数目和峰位置通过四阶导数光谱确认,多次拟合使残差最小。所有数据均为5次重复的平均值±标准误差。利用Origin 9进行统计分析和作图,统计方法采用One-way ANOVA,进行Turkey多重比较(P<0.01)。
-
毛竹叶片和茎秆之间光合色素存在极显著差异(表1),在茎秆顶部,叶绿素和类胡萝卜素的质量分数最低,比叶片分别低了93.4%和96.3% (P<0.01)。随着茎秆的发育成熟,叶绿素和类胡萝卜素开始大量累积,在茎秆基部达到最高,比叶片分别低了30.6%和47.6%(P<0.01)。茎秆叶绿素a/b均比叶片小,在茎秆成熟过程中,叶绿素a/b逐渐升高。茎秆基部和中部叶绿素a/b与叶片之间无显著差异,茎秆顶部叶绿素a/b比叶片高46.5% (P<0.01)。
表 1 毛竹叶和茎秆的光合色素质量分数
Table 1. Pigment content in leaves and stems of Ph. edulis
位置 质量分数/(μg·g−1) 叶绿素a/类胡萝卜素 叶绿素a/b 叶绿素a 叶绿素b 总叶绿素 类胡萝卜素 叶 311.60±0.07 A 89.30±0.01 A 400.90±0.08 A 96.00±0.01 A 3.23±0.25 A 3.46±0.27 B 茎秆基部 158.00±1.09 B 52.10±0.20 B 210.10±1.28 B 66.60±0.48 B 2.37±0.06 B 3.03±0.10 B 茎秆中部 64.90±0.29 C 17.00±0.09 C 81.90±0.23 C 28.90±0.17 C 2.25±0.15 B 3.83±0.35 B 茎秆顶部 12.50±0.01 D 2.50±0.01 D 15.00±0.01 D 6.30±0.09 D 2.02±0.31 B 5.07±0.23 A 说明:同列不同大写字母表示差异极显著(P<0.01) -
采用非变性BN-PAGE电泳技术分析了毛竹叶片和茎秆类囊体膜蛋白复合物(图1)。毛竹叶片BN凝胶中蛋白复合物包括2种LHCⅡ-PSⅡ超复合物、约600 kDa的PSⅡ核心二聚体和PSⅠ、不同分子量的ATP合酶(ATP-synthase)组件、约290 kDa的PSⅡ核心单体、缺少CP43亚基的PSⅡ核心单体、约140 kDa的游离LHCⅡ三聚体和游离LHCⅡ单体。通过对比叶片类囊体膜蛋白复合物,茎秆BN凝胶中明显的条带有LHCⅡ-PSⅡ超复合物、PSⅡ核心二聚体几乎与PSⅠ相连、ATP合酶、PSⅡ核心单体和LHCⅡ单体。在叶片BN凝胶中,最丰富的捕光色素蛋白复合物是LHCⅡ三聚体;而在茎秆BN凝胶中,最多的则是LHCⅡ单体。茎秆基部PSⅡ单体和二聚体与叶片相似,而缺少CP43亚基的PSⅡ单体的颜色明显比叶片淡。随着茎秆的成熟,PSⅡ单体和二聚体越显著。
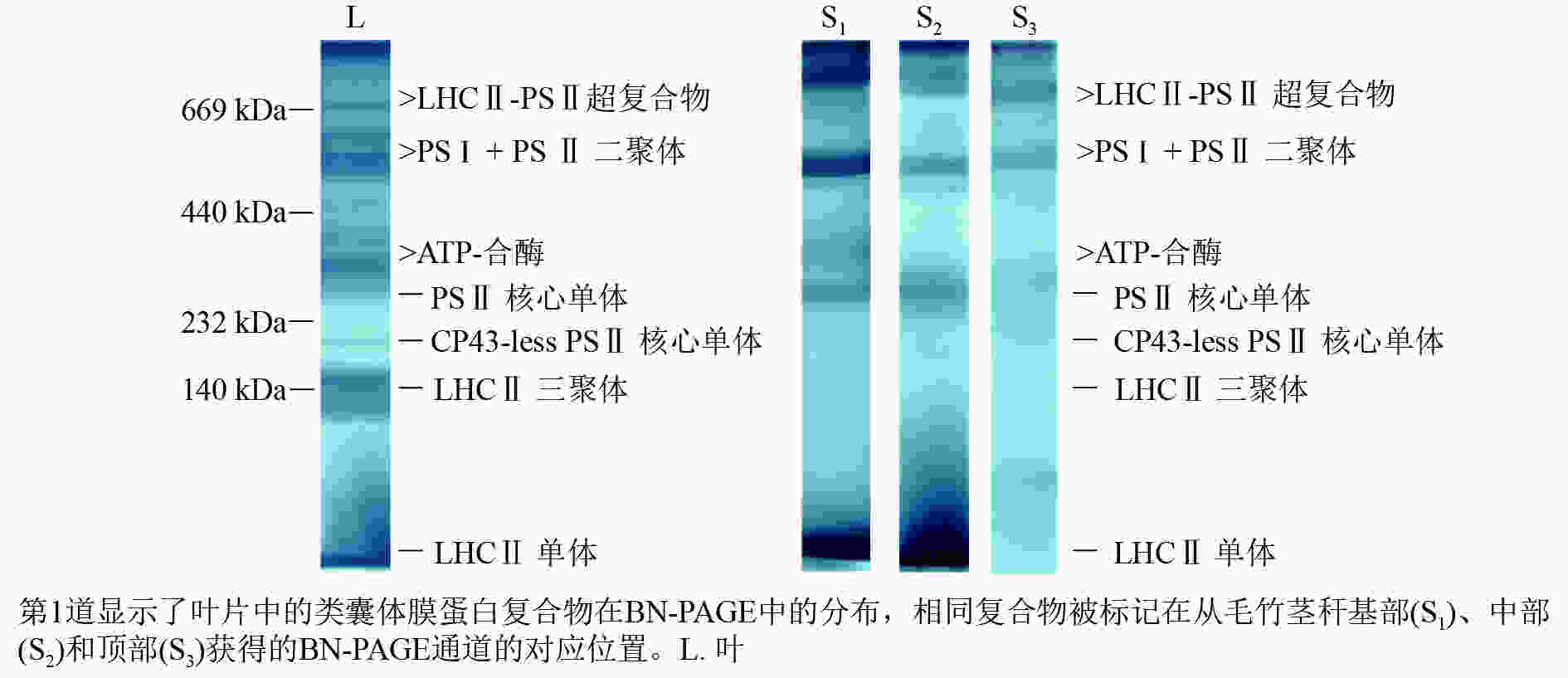
图 1 毛竹类囊体膜蛋白BN-PAGE电泳图
Figure 1. Blue-native gel electrophoresis analysis of Ph. edulis thylakoid membrane protein complexes
为了更好地比较两者差异,将第1向分离的胶条切下,进行第2向Tricine-SDS-PAGE电泳。BN-PAGE第1向电泳分离了类囊体膜大分子蛋白复合物,经第2向电泳分离得到各小亚基(图2),参考RANTALA等[27]的研究结果,比较光系统发育情况。经过考马斯亮蓝染液的染色补充,显示了与CP47、CP43、D2、D1和LHCⅡ亚基对应的位置。蛋白复合物对应于:LHCⅡ-PSⅡ超复合物、PSⅡ核心二聚体、PSⅡ核心单体、LHCⅡ三聚体和LHCⅡ单体。ATP合酶在第2向电泳中分离得到57 kDa的ATPα和55 kDa的ATPβ。毛竹叶片和茎秆基部PSⅠ经第2向电泳分离得到PsaA/B和PsaD,茎秆中部得到PsaA/B,但在茎秆顶部的胶体中没有明显地显示出PsaA/B。
-
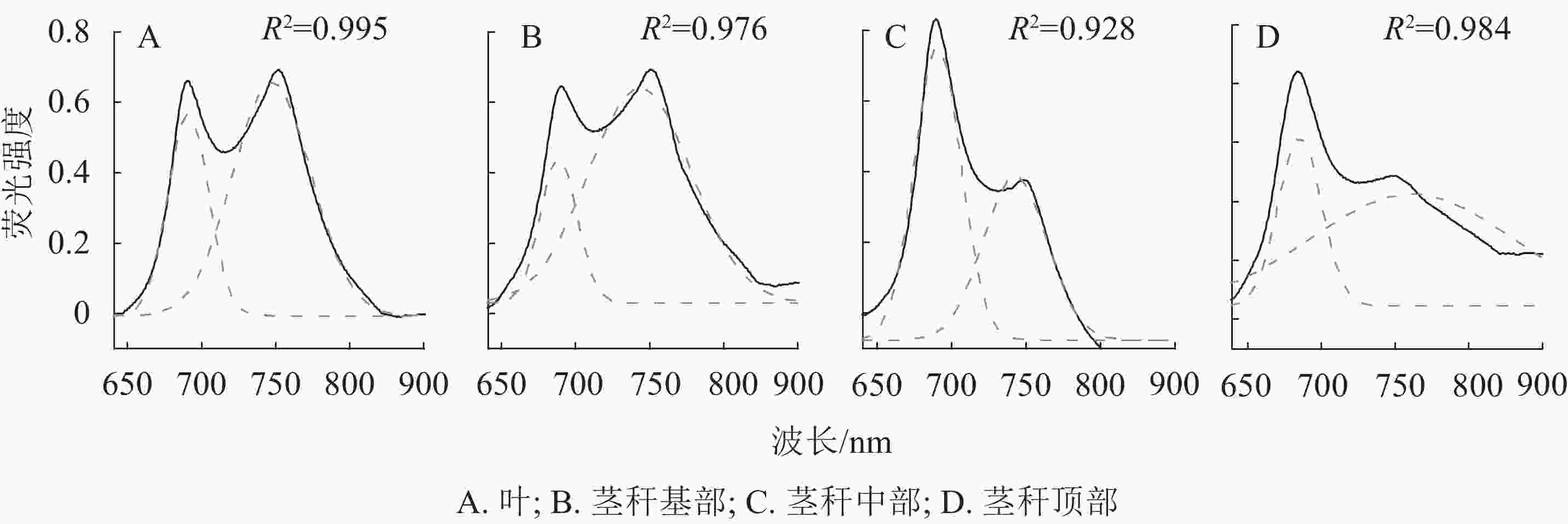
如图3A所示:以毛竹叶片光谱为参考,毛竹叶片77 K荧光发射光谱呈典型的M型,分别对应于PSⅡ (685 nm)和PSⅠ(745 nm)。茎秆荧光发射光谱被归一化为最大值,以便于茎秆相互之间的比较以及与毛竹叶片的比较。茎秆基部光谱与毛竹叶片的基本一致,茎秆中部在685 nm对应PSⅡ,荧光强度比毛竹叶片高;在745 nm对应PSⅠ,荧光强度最低。茎秆顶部在680 nm处出现蓝移现象。此外,荧光强度与茎秆中部一致;在745 nm处荧光强度比毛竹叶片低且主峰不明显。

图 3 毛竹叶和茎秆的77 K荧光发射光谱和四阶导数光谱
Figure 3. 77 K fluorescence emission spectrum and fourth-derivative spectrum from leaves and stems of Ph. edulis
对毛竹叶片和茎秆的77 K荧光发射光谱进行四阶导数处理(图3B)。红光区毛竹叶片与茎秆基部以及中部的四阶导数光谱基本一致,主峰均在685 nm处,各有1个肩峰均在653 nm处;茎秆顶部的四阶导数光谱出现明显的蓝移现象。主峰在680 nm处,在650 和710 nm处有2个肩峰。毛竹叶片和茎秆的四阶导数光谱在远红光区差异很大,毛竹叶片和茎秆基部及中部主峰在745 nm处,在725和765 nm处有2个肩峰;茎秆顶部的主峰在741 nm处,在725 和750 nm处有2个肩峰。
在77 K荧光发射光谱范围内,通过高斯函数解析出2个高斯光谱组分(表2,图4)。在红光区,毛竹叶片和茎秆的荧光峰都在685 nm左右;在远红光区,毛竹叶片和中下部茎秆的荧光峰在740 nm左右,但茎秆顶部荧光峰在756 nm处,出现明显红移。基部和顶部的2个解析峰面积比(A1/A2)、峰高比值(H1/H2)和2个荧光峰的半峰宽比(W1/W2)分别比毛竹叶片低46.7%和9.9%、30.8%和41.1%、22.1%和35.6% (P<0.01);中部的A1/A2、H1/H2、W1/W2分别比毛竹叶片高191.1%、40.4%和109.3% (P<0.01)。
表 2 毛竹叶和茎秆的77 K荧光发射光谱高斯解析结果
Table 2. Results of Gaussian decomposition of 77 K fluorescence spectra for leaves and stems of Ph. edulis
峰 主峰1 主峰2 峰面积1 峰面积2 半峰宽1 半峰宽2 峰高1 峰高2 峰面积比 峰高比 半峰宽比 叶 687 741 18.94 42.20 26.38 50.87 0.57 0.66 0.45 0.52 0.86 茎秆基部 685 738 12.29 51.10 23.89 66.76 0.41 0.61 0.24 0.36 0.67 茎秆中部 688 768 31.39 23.92 30.32 41.30 0.83 0.46 1.31 0.73 1.80 茎秆顶部 684 756 20.09 61.85 28.09 130.48 0.57 0.38 0.32 0.22 1.50 -
光在植物生长中具有特殊作用,除了作为一种能源控制着光合作用,还作为一种触发信号影响着植物生长[28]。ALEXANDER等[29]研究发现:樟子松Pinus sylvestris var. mongolica针叶和树皮中叶绿素a/b和类胡萝卜素组成相似。本研究结果显示:茎秆受到光照后,色素大量形成。茎秆基部和中部叶绿素a/b相似,但茎秆顶部叶绿素a/b显著高于毛竹叶片。这可能因为中上部有笋衣包裹,受到光照强度随着茎秆节间的升高而减弱,为了适应这种环境,茎秆叶绿体中会有较高的叶绿素a/b。
在芒果Mangifera indica等绿色肉质水果中,PSⅡ的效率普遍较高,与叶片的效率相当[30-31]。FERRONI等[32]研究发现,成熟果实中CP43-less PSⅡ核心单体含量比叶片高,成熟果实中LHCⅡ-PSⅡ超复合物的含量比叶片低。BONORA等[33]发现类囊体在成熟早期发生,随后在成熟后期大量积累类胡萝卜素。PSⅡ反应中心由蛋白D1和D2组成[34],两侧是内周捕光天线蛋白CP47和CP43[35],大多数与PSⅡ相关的叶绿素都存在于外周捕光天线(LHCⅡ)复合体中[36]。BARSAND等[37]发现番茄Solanum lycopersicon叶绿体向色质的转变与光系统发生机制的破坏(囊泡运输、为类囊体生物合成提供材料、光系统组装)是一致的,会导致光反应蛋白的减少。毛竹叶片PSⅡ经分离得到了反应中心蛋白D2、D1、内周天线蛋白CP47、CP43以及大量外周捕光天线蛋白,叶片PSⅡ发育完全;茎秆PSⅡ经分离也得到了D2、D1、CP47、CP43蛋白,但外周捕光天线蛋白明显比叶片少,从茎秆基部到顶部外周捕光天线蛋白数量显著减少。表明毛竹茎秆中PSⅡ核心复合物已形成,随着茎秆发育,笋衣逐渐脱落,茎秆受到光照加强,色素大量形成,捕光天线蛋白逐渐增多,使得PSⅡ复合体发育更完整。
SMART等[38]发现:psaA或psaB基因失活都将导致PSⅠ复合物在类囊体中缺失,表明PsaA或PsaB不能单独形成二聚体,而PsaA/B异二聚体的存在是整个PSⅠ复合物组装所必需的。PSⅠ的外周蛋白PsaD和PsaE位于类囊体基质侧形成一个凸点。有研究[39]显示:PsaD、PsaE和Fd之间有互作关系,普遍认为PsaD通过与带负电荷Fd之间的静电互作而为Fd提供必要的结合位点,同时PsaD也是PSⅠ中PsaC和PsaE正确组装所必需的。毛竹叶片PSⅠ经分离得到了PsaA/B和PsaD小亚基,叶片PSⅠ已发育完全;茎秆基部发现了PsaA/B和PsaD亚基,PSⅠ核心复合物已形成,小亚基正在整合到PSⅠ核心复合物上;茎秆中部发现PsaA/B,但未发现其他小亚基,这可能是因为茎秆中部PSⅠ核心复合物在组装过程中,小亚基都呈现游离状态,在提取类囊体膜蛋白复合物时,这些小亚基未能够完整提取;茎秆顶部未发现PsaA/B和小亚基,PSⅠ核心复合物还未组装。这表明茎秆PSⅠ的发育是从基部开始的,随着笋衣脱落,裸露的茎秆受到光照,色素大量形成,PSⅠ核心复合物开始组装完整。
植物叶绿体内不同的色素蛋白复合物发射的荧光构成了叶绿体的荧光发射光谱[40],而这些蛋白复合物具有不同的发射荧光峰位[41]。茎秆基部在红光区的77 K荧光发射光谱与毛竹叶片基本一致,这说明光照不足对毛竹叶片与成熟茎秆PSⅡ核心复合物的组成无显著影响。红光区内的肩峰主要是CP47、CP43和LHCⅡ引起的[42];远红光区附近的肩峰是由PSⅠ反应中心和LHCⅠ引起的[43]。在红光区内茎秆顶部的最大峰出现了蓝移现象,这可能是由于茎秆顶部被笋衣包裹,光照不足会导致CP47等亚基含量减少[44];也可能是因为PSⅡ中的CP47、D1、D2等色素蛋白的二级结构以及色素分子的空间位置发生了改变,导致蓝移现象[45]。毛竹叶片的叶绿素a和类胡萝卜素远远高于茎秆,而毛竹叶片荧光强度比茎秆低。这可能是因为PSⅡ核心复合物只结合有叶绿素a和β-胡萝卜素(β-Car),当激发光激发PSⅡ核心复合物时,稳态发射光谱显示随着β-胡萝卜素分子吸收强度的增加,向叶绿素a分子进行能量传递的荧光耗散就越少,其发射荧光强度就降低[13]。远红光区内的光谱特征可能只由于毛竹叶片和茎秆基部叶绿体中的PSⅠ核心复合物基本都已形成,但茎秆中部和顶部 PSⅠ核心复合物还未完全形成,还有较多的的LHCⅠ和PSⅠ-LHCⅠ,所以光谱中出现肩峰。
综上所述,在毛竹茎秆成熟早期,PSⅡ核心复合体已形成,随着茎秆发育,笋衣逐渐脱落,色素大量合成,内周天线蛋白CP47和CP43以及外周捕光天线蛋白逐渐形成,PSⅡ的77 K发射光谱荧光强度逐渐减小;同时,茎秆受到光照后PSⅠ核心蛋白PsaA和PsaB开始形成,逐渐组装合成PSⅠ核心复合体,PSⅠ的77 K发射峰荧光强度逐渐增大。
BN-PAGE analysis of thylakoid membrane protein complex during rapid growth of Phyllostachys edulis
-
摘要:
目的 探讨毛竹Phyllostachys edulis笋竹茎秆的光合特性和光系统的发育情况。 方法 以当年生毛竹叶片和笋竹茎秆为材料,采用蓝绿温和胶电泳(BN-PAGE)分析茎秆和叶片类囊体膜蛋白,同时测定了光合色素含量和77 K低温荧光发射光谱。 结果 茎秆叶绿素和类胡萝卜素质量分数显著低于叶片(P<0.01),随着茎秆发育,叶绿素和类胡萝卜素质量分数显著升高。茎秆和叶片类囊体膜PSⅡ核心复合物较完整,捕光色素较多;叶片和茎秆基部PSⅠ核心复合物分离主要得到PsaA/B和PsaD亚基,茎秆中部得到PsaA/B,茎秆顶部未发现PsaA/B。叶片和茎秆77 K低温荧光发射光谱在685和745 nm处有2个明显主峰,四阶导数光谱出现6个极大值,主要是PSⅡ和PSⅠ核心复合物的荧光发射峰以及由PSⅡ外周捕光天线(LHCⅡ)、PSⅡ内周捕光天线(CP47)、PSⅡ内周捕光天线(CP43)、PSⅠ反应中心复合体(RCI)、PSⅠ捕光天线(LHCⅠ)的发射荧光峰引起的肩峰,其中茎秆顶部LHCⅡ和PSⅡ核心复合体的特征发射峰与叶片相比有明显蓝移现象。 结论 毛竹茎秆中PSⅡ核心复合体已形成,随着茎秆发育,笋衣逐渐脱落,色素大量合成,内周天线蛋白CP47和CP43以及外周捕光天线蛋白逐渐形成;同时,茎秆受到光照后PSⅠ核心蛋白PsaA和PsaB开始形成,逐渐组装合成PSⅠ核心复合体。图4表2参45 Abstract:Objective The objective of this research is to reveal the photosynthetic characteristics and the development of photosystem of the stem of Phyllostachys edulis. Method Blue-greengel electrophoresis (BN-PAGE) was used to analyze the thylakoid membrane proteins in stems and leaves, and the changes in pigment content and the 77 K low temperature fluorescence emission spectrum were measured. Result The content of chlorophyll and carotenoid in stems was significantly lower than that in leaves (P<0.01), and with the development of stems, the pigment content increased significantly. The core complex of PSⅡ in the thylakoid membrane of stems and leaves was relatively complete and there were more light-catching pigments. PsaA/B and PsaD subunits were mainly isolated from PSI core complex in leaves and the stem base, and PsaA/B was obtained from the middle of stem, but no PsaA/B was found at the top of stem. There were two obvious main peaks at 685 and 745 nm in the 77 K low temperature fluorescence emission spectrum of leaves and stems, and six maximum values in the fourth derivative spectrum, which were mainly the fluorescence emission peaks of the core complex of PSⅡ and PSⅠ, and the shoulder peak caused by the emission fluorescence peak of the PSⅡ peripheral light catching antenna (LHC Ⅱ), PSⅡ inner peripheral light catching antenna (CP47), PSⅡ inner peripheral light catching antenna (CP43), PSⅠ reaction center complex (RCI), and PSI light catching antenna (LHC Ⅰ). The characteristic emission peak of LHC Ⅱ and PSⅡ core complex at the top of stem had obvious blue shifts compared with that of leaves. Conclusion The core complex of PSⅡ in stems of Ph. edulis has been formed. With the development of stems, the bamboo shoot coat gradually falls off, the pigments are synthesized in large amount, the inner antenna proteins CP47 and CP43 and the peripheral light-catching complex are formed gradually. Meanwhile, the PSⅠ core proteins PsaA and PsaB begin to form after the stems are exposed to light, and the core complex of PSⅠ is gradually assembled and synthesized. [Ch, 4 fig. 2 tab. 45 ref.] -
Key words:
- Phyllostachys edulis /
- thylakoid membrane protein /
- photosystem /
- emission fluorescence
-
表 1 毛竹叶和茎秆的光合色素质量分数
Table 1. Pigment content in leaves and stems of Ph. edulis
位置 质量分数/(μg·g−1) 叶绿素a/类胡萝卜素 叶绿素a/b 叶绿素a 叶绿素b 总叶绿素 类胡萝卜素 叶 311.60±0.07 A 89.30±0.01 A 400.90±0.08 A 96.00±0.01 A 3.23±0.25 A 3.46±0.27 B 茎秆基部 158.00±1.09 B 52.10±0.20 B 210.10±1.28 B 66.60±0.48 B 2.37±0.06 B 3.03±0.10 B 茎秆中部 64.90±0.29 C 17.00±0.09 C 81.90±0.23 C 28.90±0.17 C 2.25±0.15 B 3.83±0.35 B 茎秆顶部 12.50±0.01 D 2.50±0.01 D 15.00±0.01 D 6.30±0.09 D 2.02±0.31 B 5.07±0.23 A 说明:同列不同大写字母表示差异极显著(P<0.01) 表 2 毛竹叶和茎秆的77 K荧光发射光谱高斯解析结果
Table 2. Results of Gaussian decomposition of 77 K fluorescence spectra for leaves and stems of Ph. edulis
峰 主峰1 主峰2 峰面积1 峰面积2 半峰宽1 半峰宽2 峰高1 峰高2 峰面积比 峰高比 半峰宽比 叶 687 741 18.94 42.20 26.38 50.87 0.57 0.66 0.45 0.52 0.86 茎秆基部 685 738 12.29 51.10 23.89 66.76 0.41 0.61 0.24 0.36 0.67 茎秆中部 688 768 31.39 23.92 30.32 41.30 0.83 0.46 1.31 0.73 1.80 茎秆顶部 684 756 20.09 61.85 28.09 130.48 0.57 0.38 0.32 0.22 1.50 -
[1] BIRKHOLD K T, KOCH K E, DARNELL R L. Carbon and nitrogen economy of developing rabbiteye blueberry fruit [J]. J Am Soc Hortic Sci, 1992, 117: 139 − 145. [2] KOZLOWSKI T T. Carbohydrate sources and sinks in woody plants Ⅱ carbohydrate sources and sinks in woody plants [J]. Botl Rev, 1992, 58(2): 107 − 222. [3] NILSEN E T. 10-stem photosynthesis: extent, patterns, and role in plant carbon economy[M]// GARTNER B L. Plant Stems. [s.l.]: Academic Press, 1995: 223 − 240. [4] MANETAS Y. Photosynthesizing in the rain: beneficial effects of twig wetting on corticular photosynthesis through changes in the periderm optical properties [J]. Flora, 2004, 199(4): 334 − 341. [5] SUN Q, YODA K, SUZUKI M, et al. Vascular tissue in the stem and roots of woody plants can conduct light [J]. J Exp Bot, 2005, 54(387): 1627 − 1635. [6] HIBBERD J M, QUICK W P. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants [J]. Nature, 2002, 415(6870): 451 − 454. [7] 占东霞, 张超, 张亚黎, 等. 膜下滴灌水分亏缺下棉花开花后非叶绿色器官光合特性及其对产量的贡献[J]. 作物学报, 2015, 41(12): 1880 − 1887. ZHAN Dongxia, ZHANG Chao, ZHANG Yali, et al. Photosynthetic characteristics after flowering and contribution of non-leaf green organs of cotton to yield under mulching-drip irrigation with water deficiency [J]. Acta Agron Sin, 2015, 41(12): 1880 − 1887. [8] 胡锋, 黄俊丽, 秦峰, 等. 植物叶绿体类囊体膜及膜蛋白研究进展[J]. 生命科学, 2011, 23(3): 291 − 298. HU Feng, HUANG Junli, QIN Feng, et al. Progress in chloroplast thylakoid membrane and membrane proteins [J]. Chin Bull Life Sci, 2011, 23(3): 291 − 298. [9] WOLLMAN F A, MINAI L, NECHUSHTAI R. The biogenesis and assembly of photosynthetic proteins in thylakoid membranes [J]. Biochim Biophysic Acta, 1999, 1411(1): 21 − 85. [10] SHEWRY P R. Biochemistry & molecular biology plants[M]//BUCHANAN B B, GRUISSEM W, JONES R L. Biochemistry & Molecular Biology Plants. [s.l.]: Springer Nature, 2001: 105 − 106. [11] FRISO G, GIACOMELLI L, YTTERBERG A J, et al. In-depth analysis of the thylakoid membrane proteome of Arabidopsis thaliana chloroplasts: new proteins, new functions, and a plastid proteome database [J]. Plant Cell, 2004, 16(2): 478 − 499. [12] 高荣孚, 郑彩霞, 童年, 等. 高等植物光系统Ⅰ的研究(Ⅰ):叶绿体中存在2种光系统Ⅰ[J]. 北京林业大学学报, 1997, 19(1): 13 − 20. GAO Rongfu, ZHANG Caixia, TONG Nian, et al. Photosystem Ⅰ of higher plants(Ⅰ): two populations of PSⅠ in Chloroplasts [J]. J Beijing For Univ, 1997, 19(1): 13 − 20. [13] 蔡霞, 王水才, 贺俊芳, 等. 83 K光系统Ⅱ核心复合物不同激发的荧光光谱学[J]. 光子学报, 2007, 36(6): 1128 − 1132. CAI Xia, WANG Shuicai, HE Junfang, et al. Fluorescence spectroscopy of photosystem Ⅱ core complex with different excitation wavelengths at 83 K [J]. Acta Photonica Sin, 2007, 36(6): 1128 − 1132. [14] 周伟, 何林文, 陆勤勤, 等. 条斑紫菜类囊体膜蛋白质组双向电泳研究方法[J]. 海洋科学, 2014, 38(3): 63 − 68. ZHOU Wei, HE Linwen, LU Qinqin, et al. Two-dimensional electrophoresis of thylakoid membrane proteome in Porphyra yezoensis [J]. Marine Sci, 2014, 38(3): 63 − 68. [15] 姚洪军, 张汝民, 石玉杰, 等. 豌豆光系统Ⅰ多肽HPCE分离特性的研究[J]. 内蒙古农业大学学报, 2006, 27(1): 38 − 42. YAO Hongjun, ZHANG Rumin, SHI Yujie, et al. Isolation of photosystem Ⅰ protein complex and HPCE analysis of their polypeptides [J]. J Inner Mong Agric Univ, 2006, 27(1): 38 − 42. [16] 温星, 程路芸, 李丹丹, 等. 毛竹叶片发育过程中光合生理特性的变化特征[J]. 浙江农林大学学报, 2017, 34(3): 437 − 442. WEN Xing, CHENG Luyun, LI Dandan, et al. Photosynthetic characteristics in the development process of Phyllostachys edulis [J]. J Zhejiang A&F Univ, 2017, 34(3): 437 − 442. [17] 王星星, 刘琳, 张洁, 等. 毛竹出笋后快速生长期内茎秆中光合色素和光合酶活性的变化[J]. 植物生态学报, 2012, 36(5): 456 − 462. WANG Xingxing, LIU Lin, ZHANG Jie, et al. Photosynthetic characteristics in the development process of Phyllostachys edulis [J]. Chin J Plant Ecol, 2012, 36(5): 456 − 462. [18] 孙建飞, 翟建云, 马元丹, 等. 毛竹快速生长期茎秆不同节间光合色素和光合酶活性的差异[J]. 植物学报, 2018, 53(6): 773 − 781. SUN Jianfei, ZHAI Jianyun, MA Yuandan, et al. Differences in photosynthetic pigments and photosynthetic enzyme activities in different internodes of Phyllostachys edulis during rapid growth stage [J]. Chin Bull Bot, 2018, 53(6): 773 − 781. [19] 程路芸, 温星, 马丹丹, 等. 毛竹快速生长过程中碳水化合物的时空变化[J]. 浙江农林大学学报, 2017, 34(2): 261 − 267. CHENG Luyun, WEN Xing, MA Dandan, et al. Spatial and temporal change of carbohydrates during rapid growth processes of Phyllostachys edulis [J]. J Zhejiang A&F Univ, 2017, 34(2): 261 − 267. [20] 翟建云, 孙建飞, 马元丹, 等. 毛竹快速生长期茎秆不同节间碳水化合物代谢的变化[J]. 竹子学报, 2018, 37(1): 42 − 48. ZHAI Jianyun, SUN Jianfei, MA Yuandan, et al. Changes of carbohydrates metabolism in different internodes of Phyllostachys edulis during rapid growth period [J]. J Bamboo Res, 2018, 37(1): 42 − 48. [21] CUI Kai, HE Caiyun, ZHANG Jianguo, et al. Temporal and spatial profiling of internode elongation-associated protein expression in rapidly growing culms of bamboo [J]. J Proteome Res, 2012, 11(4): 2492 − 2507. [22] PENG Zhenhua, LU Ying, LI Lubin, et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla) [J]. Nat Gen, 2013, 45: 456 − 461. [23] ARNON D I. Copper enzymes in isolated chloroplasts: Polyphenol Beta vulgaris [J]. Plant Physiol, 1959, 24(1): 1 − 15. [24] LICHTENTHALER H K. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes [J]. Methods Enzymol, 1989, 148(1): 350 − 382. [25] 陈登举, 高培军, 吴兴波, 等. 毛竹茎秆叶绿体超微结构及其发射荧光光谱特征的研究[J]. 植物学报, 2013, 48(6): 635 − 642. CHEN Dengju, GAO Peijun, WU Xingbo, et al. Chloroplast ultrastructure and emission fluorescence spectrum characteristics for stems of Phyllostachys edulis [J]. Chin Bull Bot, 2013, 48(6): 635 − 642. [26] SCHÄGGER H, von JAGOW G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form [J]. Anal Biochem, 1991, 199(2): 223 − 231. [27] RANTALA M, PAAKKARINEN V, ARO E M. Analysis of thylakoid membrane protein complexes by blue native gel electrophoresis [J]. J Visualized Exp, 2018, 139: 1 − 6. [28] 杜建芳, 廖祥儒, 叶步青, 等. 光质对油菜幼苗生长及抗氧化酶活性的影响[J]. 植物学报, 2002, 19(6): 743 − 745. DU Jianfang, LIAO Xiangru, YE Buqing, et al. Effect of light quality on the growth and antioxidant enzyme activities of rape seedings [J]. Chin Bull Bot, 2002, 19(6): 743 − 745. [29] ALEXANDER G, IVANOV, MARIANNA K, et al. Characterization of the photosynthetic apparatus in cortical bark chlorenchyma of Scots pine [J]. Planta, 2006, 223(6): 1165 − 1177. [30] CARRARA S, PARDOSSI A, SOLDATINI G F, et al. Photosynthetic activity of ripening tomato fruit [J]. Photosynthetica, 2001, 39(1): 75 − 78. [31] RANJAN S, SINGH R, SONI D K, et al. Photosynthetic performance of Jatropha curcas fruits [J]. Plant Physiol Biochem, 2012, 52: 66 − 76. [32] FERRONI L, PANTALEONI L, BALDISSEROTTO C, et al. Low photosynthetic activity is linked to changes in the organization of photosystemⅡ in the fruit of arum italicum [J]. Plant Physiol Biochem, 2013, 63: 140 − 150. [33] BONORA A, PANCALDI S, GUALANDRI R, et al. Carotenoid and ultrastructure variations in plastids of Arum italicum Miller fruit during maturation and ripening [J]. J Exp Bot, 2000, 51(346): 873 − 884. [34] BARBER J. Photosystem Ⅱ: a multisubunit membrane protein that oxidises water [J]. Curr Opinion Struct Biol, 2002, 12(4): 523 − 530. [35] FERREIRA K N, IVERSON T M, MAGHLAOUI K. Architecture of the photosynthetic oxygen-evolving center [J]. Sci, 2004, 303(5665): 1831 − 1838. [36] KUHLBRANDT W, WANG D N. Atomic model of plant light-harvesting complex by electron crystallography [J]. Nature, 1994, 367: 614 − 621. [37] BARSAN C, ZOUINE M, MAZA E, et al. Proteomic analysis of chloroplast-to-chromoplast transition in tomato reveals metabolic shifts coupled with disrupted thylakoid biogenesis machinery and elevated energy-production components [J]. Plant Physiol, 2012, 160(2): 708 − 725. [38] SMART L B, ANDERSON S L, MCINTOSH L. Targeted genetic inactivation of the photosystem Ⅰ reaction center in the Cyanobacterium synechocystis sp. Pcc 6803 [J]. Embo J, 1992, 10(11): 3289 − 3296. [39] LI Ning, ZHAO Jindong, WARREN P V, et al. PsaD is required for the stable binding of PsaC to the photosystemⅠcore protein of Synechococcus sp. Pcc 6301 [J]. Biochemistry, 1991, 30(31): 7863 − 7872. [40] GOVINDJEE E. Sixty-three years since kautsky: chlorophyll a fluorescence [J]. Aust J Plant Physiol, 1995, 22(2): 131 − 160. [41] ANDREEVA A, ABAROVA S, STOITCHKOVA K, et al. Selective photobleaching of chlorophylls and carotenoids in photosystem Ⅰ particles under high-light treatment [J]. Photochem Photobiol, 2007, 83(6): 1301 − 1307. [42] ANDREEVA A, STOITCHKOVA K, BUSHEVA M, et al. Changes in the energy distribution between chlorophyll-protein complexes of thylakoid membranes from pea mutants with modified pigment content(Ⅰ) changes due to the modified pigment content [J]. J Photochem Photobio B-Biol, 2003, 70(3): 153 − 162. [43] JENNINGS R C, ZUCCHELLI G, CROCE R, et al. The photochemical trapping rate from red spectral states in PSI-LHCI is determined by thermal activation of energy transfer to bulk chlorophylls [J]. Biochim Biophysic Acta, 2003, 1557(1/3): 91 − 98. [44] BERTAMINI M, MUTHUCHELIAN K, NEDUNCHEZHIAN N. Shade effect alters leaf pigments and photosynthetic responses in norway spruce (Picea abies) grown under field conditions [J]. Photosynthetica, 2006, 44(2): 227 − 234. [45] 蔡霞, 王水才, 贺俊芳, 等. 温度对PSⅡ CP47/D1/D2/Cyt b559复合物荧光光谱特性的影响[J]. 光子学报, 2003, 32(7): 853 − 855. CAI Xia, WANG Shuicai, HE Junfang, et al. Effect of temperature on the fluorescence spectrum characteristics of the PSⅡ reaction center CP47/D1/D2/Cyt b559 complex [J]. Acta Photonica Sin, 2003, 32(7): 853 − 855. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20190398






 下载:
下载: