-
梅花Prunus mume是中国十大传统名花,在中国有3 000多年栽培历史,江南地区花期为2月左右[1]。梅花适应性较强,耐高温、较耐低温和干旱。在年均气温为16~23 ℃的地区生长最好,根系不耐−8 ℃以下的低温[2-3]。梅花适栽范围介于自然分布区和历史分布区之间,北界为西藏经四川至甘肃天水,陕西宝鸡、西安,河南洛阳,最后到山东烟台,通过海岸线与自然分布区相接,以长江流域为集中赏梅地带[2, 4]。在华北、东北和西北等北方地区不能露地越冬,在江南地区,春季的极端低温(低于−3 ℃)则会对花(蕾)造成伤害,极大地影响观赏价值。因此,抗寒育种一直是梅花育种的重要方向[4-6]。WRKY转录因子是一类主要存在于植物中的锌指型转录因子,在植物响应生物胁迫与非生物胁迫的过程中起着重要作用。根据WRKY结构域数量和锌指结构特征,WRKY家族可分为3类:I类包含2个WRKY结构域和1个CX4−5CX22−23 HXH (C2H2)型锌指结构;Ⅱ类包含1个WRKY结构域和1个CX4−5CX22−23HXH (C2H2)型锌指结构;Ⅲ类包含1个WRKY结构域和1个CX7CX23HXC (C2HC)型锌指结构[7]。WRKY家族参与了广泛的生物过程,包括种子萌发、植物发育和植物激素信号传递等[8]。研究表明:WRKYs最重要的功能之一是参与防御非生物胁迫[9],可显著提高小麦Triticicum aestivum[10]、水稻Oryza sativa[11]等的耐寒性和香蕉Musa acuminate[12]、棉花Gossypium hirsutum[13]等的抗旱性。近年来,随着梅花基因组正式公布[14],关于梅花抗寒和抗旱基因的挖掘和研究逐渐展开,这为深入研究梅花抗寒、抗旱等机制提供了重要的基础。梅花中共鉴定出58个WRKY成员,PmWRKYs在梅花的不同组织(根、茎、叶、花和果实)中有不同程度的表达,其中17个PmWRKYs可能是调控梅花抗寒性的潜在转录因子[15]。本研究以梅花品种‘骨红朱砂’‘Guhong Zhusha’为材料,采用反转录PCR(RT-PCR)技术克隆获得了2个PmWRKY2转录因子,通过生物信息学分析和同源基因序列比对,检测PmWRKY2基因在不同非生物胁迫下的表达模式,以期为后续开展WRKY转录因子在梅花抗寒和抗旱方面的作用机制研究奠定基础。
-
梅花‘骨红朱砂’来自浙江农林大学梅花种质资源圃。采集生长势一致,无病虫害的1年生枝条,插于加入清水的培养瓶中,参照PENG等方法[16]处理,湿度为50%,光周期为16 h/8 h。低温(2 ℃)处理组取样时间为0(ck)、1、2、4、6、12、24、48、72 h。采用200 mmol·L−1的甘露醇溶液模拟干旱处理,取样时间为0(ck)、3、6、12、24、36、48 h。采用100 μmol·L−1脱落酸(ABA)处理,取样时间为0(ck)、3、6、12、24、36、48 h。所有新鲜样品采集后立即用液氮速冻,保存于−80 ℃,3次生物学重复。
-
RNA的提取采用购自天津诺禾致源公司的UltraClean Polysaccharide and Phenol Plant RNA Purification Kit,方法参照试剂盒的提取说明书。cDNA的合成根据TAKARA PrimeScript™ RT Master Mix (Perfect Real Time)说明书在冰上进行。
-
通过梅花基因组和表达谱数据获得PmWRKY2-1和PmWRKY2-2序列,利用Prime 5.0设计特异性引物(表1),以‘骨红朱砂’叶片cDNA为模板,利用r-Taq DNA聚合酶进行PCR扩增。扩增条件为:95 ℃预变性5 min;95 ℃变性30 s,54 ℃退火30 s,72 ℃延伸3 min,35个循环;72 ℃延伸10 min。PCR扩增产物经切胶回收试剂盒回收后连接到pMD18-T载体(Takara公司,大连)中,转化大肠埃希菌Escherichia coli DH5α感受态细胞后挑取阳性克隆,经PCR验证后送往杭州有康科技有限公司测序。
表 1 基因克隆及表达所用引物序列
Table 1. Primers used in Gene clone and Quantitative real-time PCR
用途 引物名称 序列(5′→3′) 基因克隆 PmWRKY2-1F ATGGCTGGCATCGATGA PmWRKY2-1R CTACATCTGTGGTCCAAG PmWRKY2-2F ATGGGATTTTTAAGAACC PmWRKY2-2R CTAGTACGATTGATGACTGCTTC 实时荧光定量PCR QPmWRKY2-1F GTCCCCTTATCTGACAATACCTC QPmWRKY2-1R AAAGCGAATGAAGTATTTATGTCCT QPmWRKY2-2F TCCGTTGCTTCCTCCCAATGATGAC QPmWRKY2-2R CAAAATCTATTGGTTGTTGCTCC QPmEF1αS CGGATTCAATGTTAAGAATGTTGC QPmEF1αA AGAACTGGAGCATATCCGTTACC -
采用在线软件BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) 进行基因序列比对分析,用ORF finder在线分析开放阅读框,运用ProtParam 在线软件(http://web.expasy.org/protparam/)预测编码蛋白质的分子量、理论等电点;利用 SOPMA 在线工具分析PmWRKY2蛋白质的二级结构组成;利用WOLFPSORT在线软件预测基因的亚细胞定位;利用 DNAMAN 9.0软件对梅花PmWRKY2蛋白质与其他物种WRKY蛋白质进行比对分析;使用ClutsalX-v1.83程序进行多序列比对,然后将比对结果输入到MEGA 6.0软件中,利用邻接法(neighbor-joining, NJ)构建系统发育树,Bootstrap值取1 000次。
-
以不同处理的叶片为模板,反转录为cDNA,并进行实时荧光定量PCR。利用Prime 5.0设计PmWRKY2-1和PmWRKY2-2的特异性引物,以梅花PmEF1α为内参基因。反应体系为SYBR Premix Ex Taq 酶(Takara,大连)10.0 μL,cDNA 2.0 μL,上下游引物(10 μm·L−1)各0.8 μL,双蒸水 6.4 μL,每个样品设置3次重复。反应程序为两步法:95 ℃预变性30 s,95 ℃变性5 s,60 ℃复性30 s,共40个循环;然后以95 ℃持续5 s,60 ℃持续1 min,95 ℃持续15 s作为溶解曲线分析程序,最后根据
$ {2^{ - \Delta \Delta {C_{\rm{t}}}}} $ 法计算目的基因的相对表达量。 -
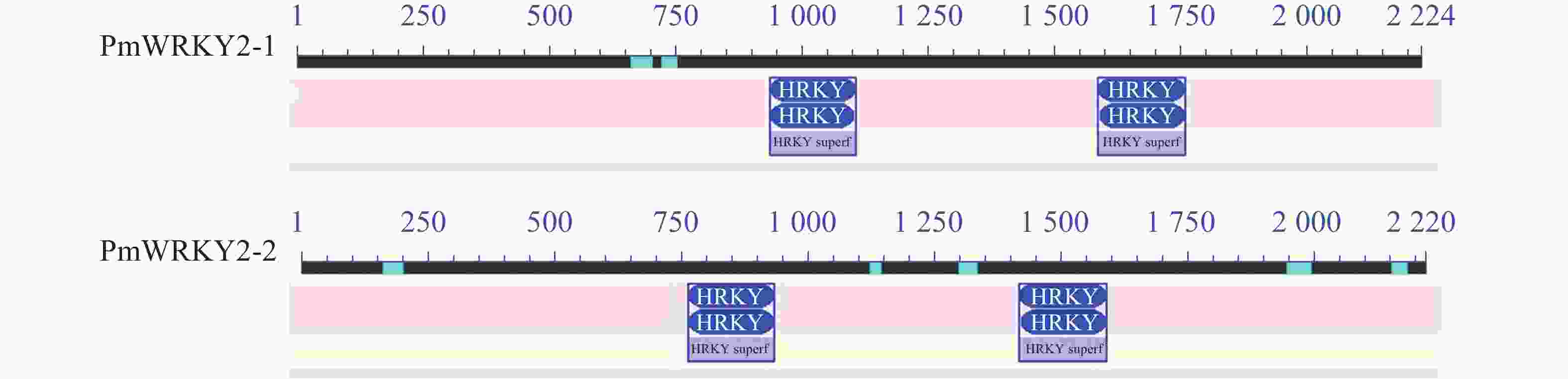
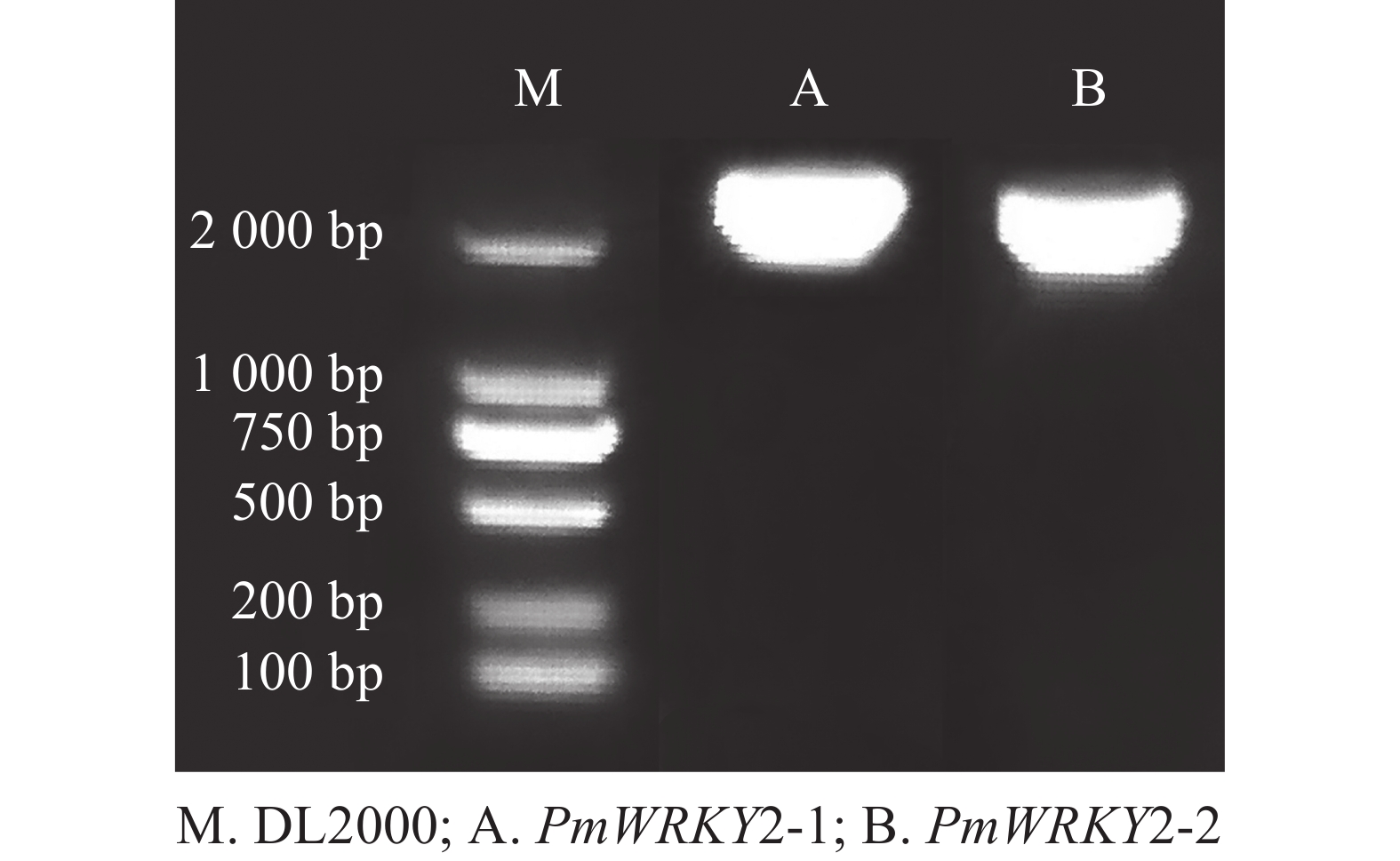
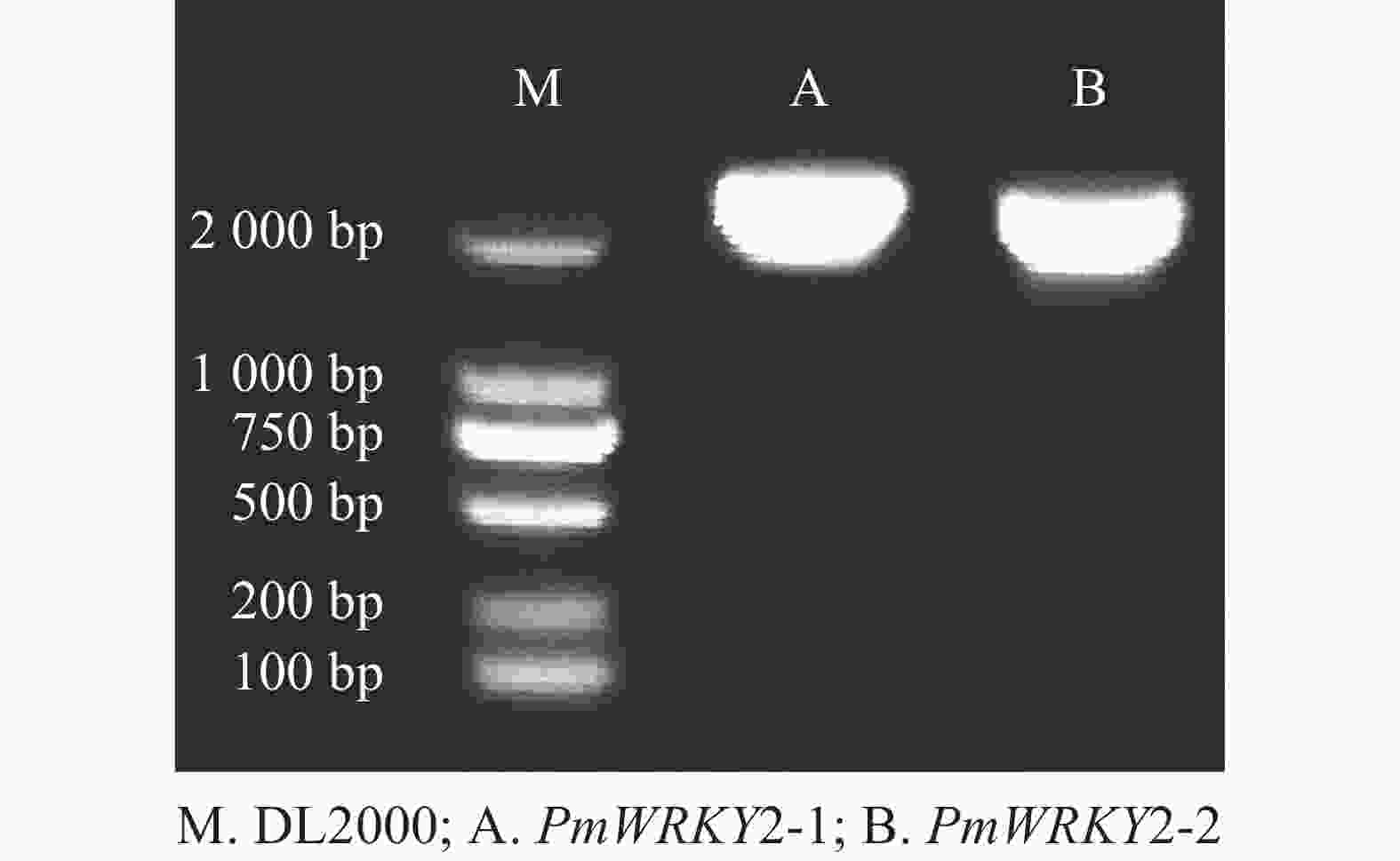
利用特异性引物进行PCR扩增,经过连接、转化、测序后获得编码序列(CDS)。测序结果显示:PmWRKY2-1和PmWRKY2-2的CDS长度分别为2 223和2 220 bp(图1),编码的氨基酸数目分别为740和739个,蛋白质分子量分别为79.94和80.98 kD,理论等电点分别为5.65和5.82。不稳定系数分别为53.93和53.82,脂肪指数分别为54.18和59.53,预测它们为不稳定蛋白质。总平均亲水系数(GRAVY)分别为−0.774和−0.743,属于亲水性蛋白质。亚细胞定位预测结果显示:PmWRKY2-1和PmWRKY2-2均位于细胞核。
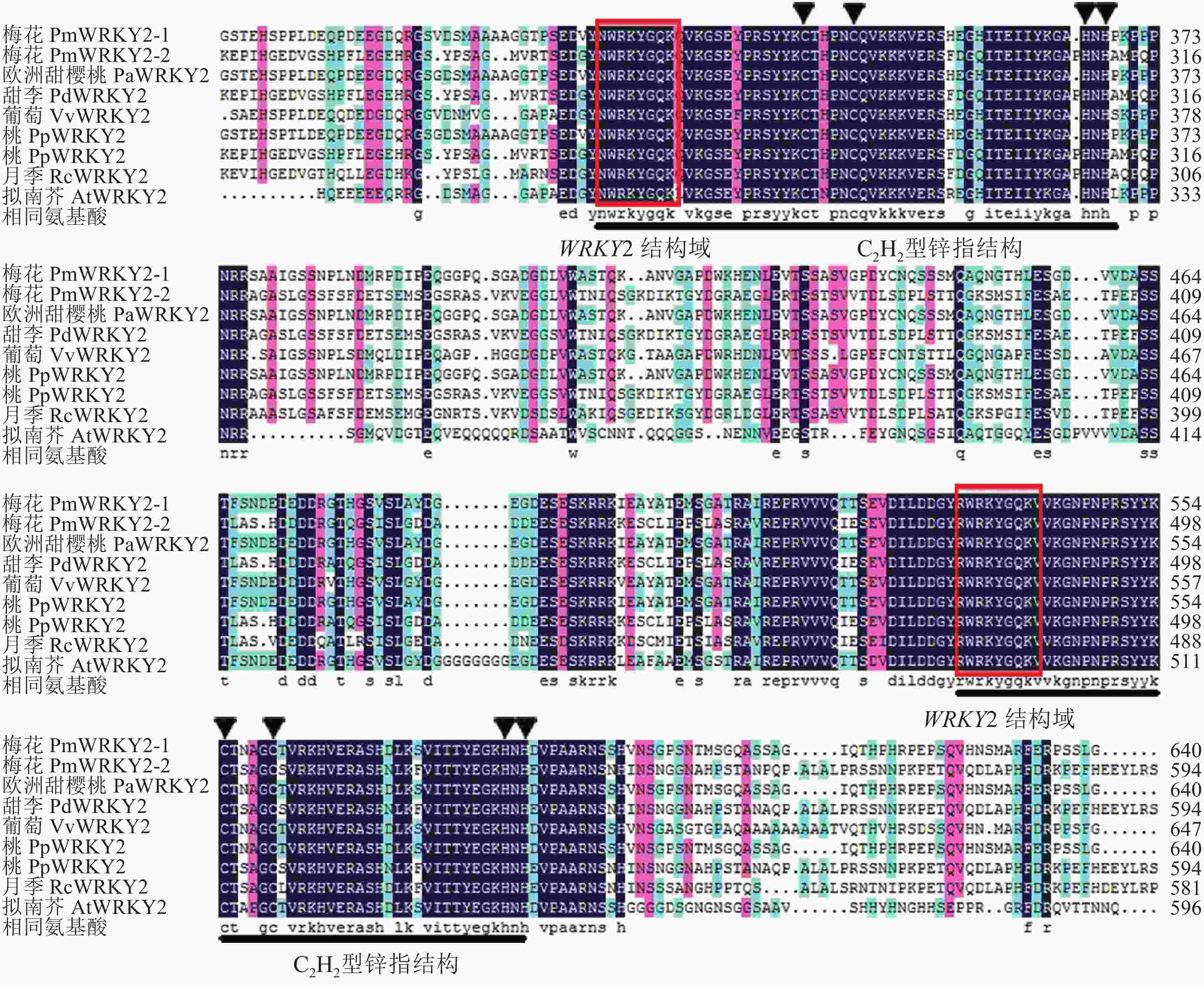
氨基酸序列比对结果显示(图2):梅花PmWRKY2-1和PmWRKY2-2的同源性仅为45.87%,与拟南芥Arabidopsis thaliana的AtWRKY2相似性分别为51.26%和32.07%;其中PmWRKY2-1与欧洲甜樱桃P. avium(XP_021826759.1)、桃P. persica(XP_007206427.1)的WRKY2同源性分别为98.65%,98.78%;PmWRKY2-2与甜李P. dulcis(XP_034218428.1)、桃(XP_007207009.2)的WRKY2同源性分别为98.51%,98.11%,与月季Rosa chinensis(XP_024188041.1)WRKY2同源性为77.97%。进一步分析发现:梅花PmWRKY2-1和PmWRKY2-2氨基酸序列与其他植物氨基酸序列一样,均包含2个WRKY结构域和1个CX4−5CX22−23 HXH(C2H2)型锌指结构,属于Group Ⅰ (图3)。
-
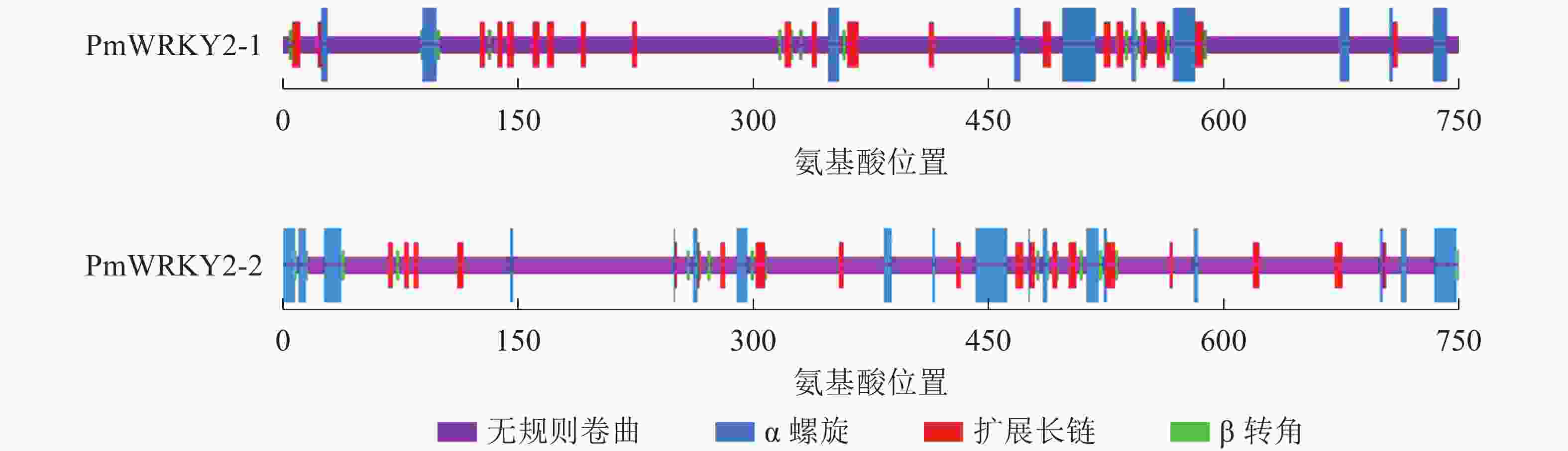
蛋白质二级结构预测结果显示(图4):PmWRKY2-1蛋白质的二级结构中包含75.68%的无规则卷曲、10.81%的α螺旋、10.41%的扩展长链和3.11%的β转角结构;PmWRKY2-2蛋白质的二级结构中包含74.02%的无规则卷曲、13.80%的α螺旋、8.80%的扩展长链和3.38%的β转角结构。
-
利用MEGA 6.0软件构建梅花PmWRKY2-1和PmWRKY2-2氨基酸序列的系统进化树(图5)。结果显示:梅花PmWRKY2-1与PmWRKY2-2的相似性较低,但与一些蔷薇科Rosaceae植物的亲缘关系都较近;其中PmWRKY2-1与桃(XP_007206427.1)、欧洲甜樱桃(XP_021826759.1)的WRKY亲缘关系较近,PmWRKY2-2与甜李(XP_034218428.1)、桃(XP_007207009.2)的WRKY氨基酸聚为一类,与麻疯树Jatropha curcas (XP_021629940.1)、酸枣Ziziphus jujube(XP_015875770.1)、蔓花生Arachis duranensis(XP_015962000.1)等SUSIBA2-Like(sugar signaling in barley)基因的氨基酸序列也有一定的相似性。
-
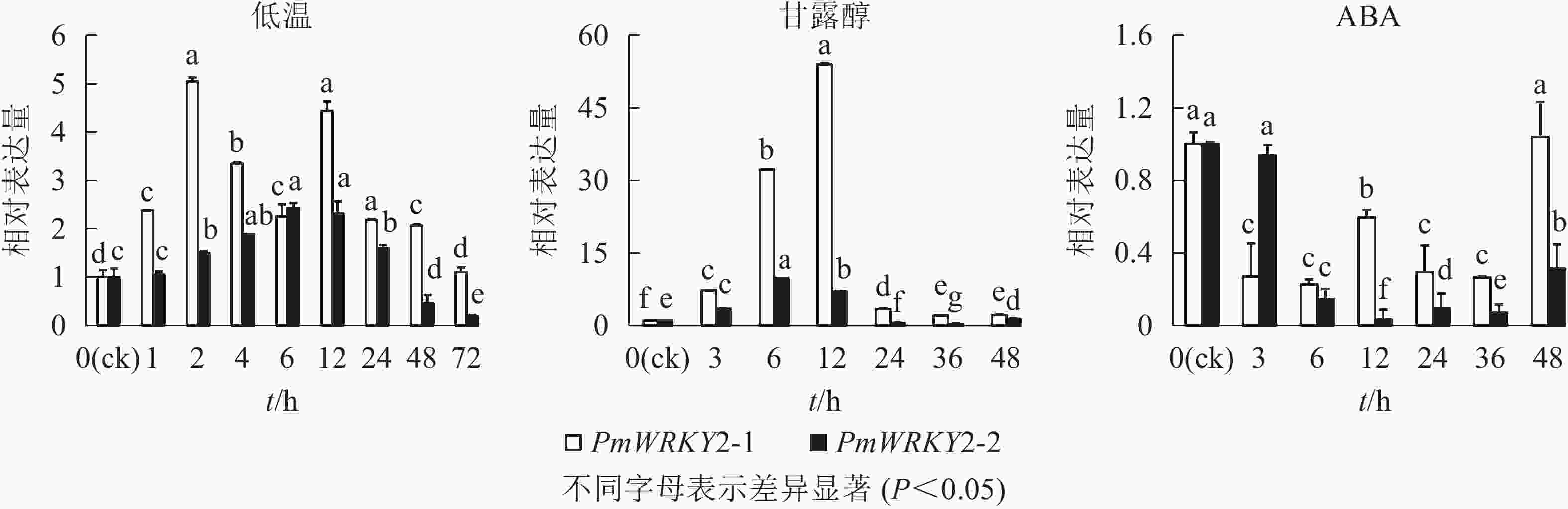
低温和干旱(甘露醇)处理后,PmWRKY2-1和PmWRKY2-2表达均发生显著变化(图6)。在低温处理下,PmWRKY2-1在2 和12 h时表达量最高,分别是对照的5.0和4.4倍,之后呈现下降的趋势;PmWRKY2-2的表达量呈现先上升后下降的趋势,在6 h时达到最大值,是对照的2.4倍。在干旱处理下,PmWRKY2-1和PmWRKY2-2的表达模式均为先上升后下降的趋势,PmWRKY2-1的表达量在12 h达到最大且为对照的53.9倍,之后便呈现下降的趋势;PmWRKY2-2的表达量在6 h处达到最大,为对照的9.7倍,之后呈现下降趋势。在脱落酸(ABA)处理下,处理48 h前,PmWRKY2-1和PmWRKY2-2均显著下调(P<0.05),说明其表达可被ABA抑制。
-
植物在生长发育的过程中会受到多种因素的影响,而低温与干旱是常见的影响植物生长发育、果实品质以及地理分布的非生物胁迫因素,严重时可能会导致植物死亡。植物在长期适应进化的过程中逐渐形成了复杂而高效的应答机制,从分子、生理、细胞和生化等多方面做出适应性调整,以抵御和适应低温、干旱等胁迫。在植物响应低温、干旱胁迫过程中,普遍存在于植物中的WRKY转录因子发挥了重要作用[17],目前已在拟南芥[7]、番茄Solanum lycopersicum[18]、玉米Zea mays [19]、苹果Malus domestica[20]、水稻[21]等大多数物种中均有报道。
本研究克隆获得的PmWRKY2-1和PmWRKY2-2基因都含有2个WRKY结构域,C端都为C2H2型锌指结构;但2个蛋白质序列的差异较大,相似性仅为45.87%;与一些蔷薇科植物的亲缘关系较近;PmWRKY2-1与拟南芥AtWRKY2的相似性为51.26%,PmWRKY2-2为32.07%。值得一提的是,PmWRKY2-2与麻风树(XP_021629940.1)、酸枣(XP_015875770.1)、蔓花生 (XP_015962000.1)等植物的SUSIBA2-Like氨基酸序列具有一定的相似性,有研究[22]报道SUSIBA2属于WRKY转录因子超家族并参与碳水化合物合成代谢。
罗昌国等[23]发现:低温处理下湖北海棠Malus hupehensis MhWRKY40b基因表达量呈现先上升后下降的趋势;低温处理下黄瓜Cucumis sativus CsWRKY46[24]和水稻OsWRKY76[11]也呈现先上升后下降的表达趋势,与本研究中梅花PmWRKY2-1和PmWRKY2-2基因对低温的响应趋势一致。干旱处理下PmWRKY2-1和PmWRKY2-2的表达量先显著上升后下降,最高表达量分别上调了约50倍和10倍。ZHU等[25]发现:拟南芥中过表达甘薯Ipomoea batatas IbWRKY2和苦荞[26]Fagopyrum tataricum FtWRKY10能提高转基因植株的抗旱性;ZHANG等[27]发现:吲哚-3-乙酸(indole-3-acetic acid)处理白车轴草Trifolium repens,其WRKY2作为干旱响应基因可以提高白车轴草的耐旱性。JIANG等[28]发现:ABI5、ABI3、ABA2和ABA3等ABA途径基因诱导拟南芥2个WRKY2的表达从而介导种子萌发和萌发后的发育停滞。本研究中,ABA处理下,梅花2个PmWRKY2基因的表达都被抑制,预测启动子序列中的PmWRKY2-1和PmWRKY2-2分别含有1个和7个ABA响应元件ABRE,推测这2个基因可能通过ABA调控低温相关基因的表达进而调控梅花的耐寒性,但这些推论还需进一步验证。
Cloning and expression analysis under adversity stress of 2 PmWRKY2 in Prunus mume
-
摘要:
目的 低温是影响梅花Prunus mume栽培应用的重要环境因素。WRKY基因是一类主要存在于植物中的转录因子,参与响应非生物胁迫等过程。了解梅花WRKY基因对非生物和脱落酸(ABA)胁迫的响应,对梅花定向育种具有重要的意义。 方法 以梅花‘骨红朱砂’Prunus mume ‘Guhong Zhusha’为材料,通过反转录PCR(RT-PCR)克隆获得了2个WRKY2转录因子,命名为PmWRKY2-1和PmWRKY2-2;采用实时荧光定量PCR(qRT-PCR)分析PmWRKY2-1和PmWRKY2-2基因在低温和干旱下的表达模式。 结果 PmWRKY2-1和PmWRKY2-2的编码区长度分别为2 223和2 220 bp,分别编码740和739个氨基酸,包含2个WRKY结构域和C2H2型锌指结构;PmWRKY2-1与PmWRKY2-2亲缘关系较远,但两者与蔷薇科Rosaceae植物欧洲甜樱桃P. avium、桃P. persica、李P. dulcis的亲缘关系较近。qRT-PCR结果显示:在低温和干旱处理下,PmWRKY2-1与PmWRKY2-2都能被诱导;脱落酸(ABA)处理后,PmWRKY2-1与PmWRKY2-2的表达显著降低。 结论 PmWRKY2-1与PmWRKY2-2可能参与调控梅花低温和干旱响应,并可能受到ABA的调控。图6表1参28 Abstract:Objective Low temperature is a main environmental factor that influences the cultivation and application of Prunus mume whereas WRKY gene is a plant-specific transcription factor which participates in the response to abiotic stress process. This study, with an investigation of how WRKY gene responds to low temperature and drought stress, is aimed to provide guidance for the directional breeding of P. mume. Method With the P. mume ‘Guhong Zhusha’ cDNA template selected as the substance, two WRKY2 genes were cloned by means of RT-PCR, named as PmWRKY2-1and PmWRKY2-2 before their expression patterns were under low temperature and in the condition of drought employing real-time quantitative PCR (qRT-PCR). Result a) PmWRKY2-1 and PmWRKY2-2, with respective coding area lengths of 2 223 and 2 220 bp, encode 740 and 739 amino acids respectively, both including 2 WRKY domains and a C2H2 zinc finger structure; b) though with a distant genetic relationshp with each other, both PmWRKY2-1 and PmWRKY2-2 had a close relationship with P. avium, P. persica and P. dulcis; c) according to the results of the real-time quantitative PCR (qRT-PCR), both PmWRKY2-1 and PmWRKY2-2 could be induced by low temperature and drought treatment And d) the expressions of PmWRKY2-1 and PmWRKY2-2 were significantly reduced after abscisic acid (ABA) treatment. Conclusion PmWRKY2-1 and PmWRKY2-2 are likely to participate in the regulation of low temperature and drought response of P. mume, yet might be subject to the regulation by ABA. [Ch, 6 fig. 1 tab. 28 ref.] -
Key words:
- Prunus mume /
- WRKY transcription factor /
- gene cloning /
- expression analysis of genes
-
表 1 基因克隆及表达所用引物序列
Table 1. Primers used in Gene clone and Quantitative real-time PCR
用途 引物名称 序列(5′→3′) 基因克隆 PmWRKY2-1F ATGGCTGGCATCGATGA PmWRKY2-1R CTACATCTGTGGTCCAAG PmWRKY2-2F ATGGGATTTTTAAGAACC PmWRKY2-2R CTAGTACGATTGATGACTGCTTC 实时荧光定量PCR QPmWRKY2-1F GTCCCCTTATCTGACAATACCTC QPmWRKY2-1R AAAGCGAATGAAGTATTTATGTCCT QPmWRKY2-2F TCCGTTGCTTCCTCCCAATGATGAC QPmWRKY2-2R CAAAATCTATTGGTTGTTGCTCC QPmEF1αS CGGATTCAATGTTAAGAATGTTGC QPmEF1αA AGAACTGGAGCATATCCGTTACC -
[1] 陈俊愉. 中国梅花[M]. 海口: 中国海南出版社, 1996. [2] 陈俊愉. 中国梅花品种图志[M]. 北京: 中国林业出版社, 1989. [3] 张启翔. 梅花及其杂交种根系抗寒性研究初报[J]. 北京林业大学学报, 1992, 14(增刊 4): 83 − 86. ZHANG Qixiang. A preliminary study on cold resistance of root system of Mei and its hybrid cultivars [J]. J Beijing For Univ, 1992, 14(suppl 4): 83 − 86. [4] 包满珠, 陈俊愉. 中国梅的变异与分布研究[J]. 园艺学报, 1994, 21(1): 81 − 86. BAO Manzhu, CHEN Junyu. Studies on the variation and distribution of Prunus mume Sieb. et Zucc. [J]. Acta Hortic Sin, 1994, 21(1): 81 − 86. [5] 包满珠. 我国川、滇、藏部分地区梅树种质资源及其开发利用[J]. 华中农业大学学报, 1993, 12(5): 498 − 501. BAO Manzhu. The germplasm resources and exploitation of Prunus mume in partial area of Sichuan, Yunnan and Tibet of China [J]. J Huazhong Agric Univ, 1993, 12(5): 498 − 501. [6] 王白坡, 钱银才, 沈湘林, 等. 实生梅开花结果特性的研究[J]. 浙江林学院学报, 1992, 9(1): 6 − 13. WANG Baipo, QIAN Yincai, SHEN Xianglin, et al. Study on flowering and fruiting charaeters of seedling-mumeplant [J]. J Zhejiang For Coll, 1992, 9(1): 6 − 13. [7] EULGEM T, RUSHTON P J, ROBATZEK S, et al. The WRKY superfamily of plant transcription factors [J]. Trends Plant Sci, 2000, 5(5): 199 − 206. [8] VIVES-PERIS V, MARMANEU D, GÓMEZ-CADENAS A, et al. Characterization of Citrus WRKY transcription factors and their responses to phytohormones and abiotic stresses [J]. Biol Plant, 2018, 62(1): 33 − 44. [9] PAN Linjie, JIANG Ling. Identification and expression of the WRKY transcription factors of Carica papaya in response to abiotic and biotic stresses [J]. Mol Boil Rep, 2014, 41: 1215 − 1225. [10] NIU Canfang, WEI Wei, ZHOU Qiqun, et al. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants [J]. Plant Cell Environ, 2012, 35: 1156 − 1170. [11] YOKOTANI N, SATO Y, TANABE S, et al. WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance [J]. J Exp Bot, 2013, 64(6): 5085 − 5097. [12] LIU Guoyin, LI Bing, LI Xiang, et al. MaWRKY80 positively regulates plant drought stress resistance through modulation of abscisic acid and redox metabolism [J]. Plant Physiol Biochem, 2020, 156: 155 − 166. [13] YAN H R, JIA H H, CHEN X B, et al. The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production [J]. Plant Cell Physiol, 2014, 55(12): 2060 − 2076. [14] ZHANG Qixiang, CHEN Wenbin, SUN Lidan, et al. The genome of Prunus mume [J]. Nat Commun, 2012, 3: 1318. doi: 10.1038/ncomms2290. [15] BAO Fei, DING Anqi, CHENG Tangren, et al. Genome-wide analysis of members of the WRKY gene family and their cold stress response in Prunus mume [J]. Genes, 2019, 10(11): 911. doi: 10.3390/genes10110911. [16] PENG Ting, GUO Cong, YANG Jie, et al. Overexpression of Mei (Prunus mume) CBF gene confers tolerance to freezing and oxidative stress in Arabidopsis [J]. Plant Cell Tissue Organ Cult, 2016, 126(3): 373 − 385. [17] 李元元, 高志强, 曹清河. 甘薯SPF1转录因子的生物信息学分析[J]. 江苏农业学报, 2017, 33(4): 760 − 767. LI Yuanyuan, GAO Zhiqiang, CAO Qinghe. Bioinformatics analysis of SPF1 transcription factor of sweet potato [J]. Jiangsu Agric Sci, 2017, 33(4): 760 − 767. [18] CHEN Lin, YANG Yang, LIU Can, et al. Characterization of WRKY transcription factors in Solanum lycopersicum reveals collinearity and their expression patterns under cold treatment [J]. Biochem Biophys Res Commun, 2015, 464(6): 962 − 968. [19] WEI Kaifa, CHEN Juan, CHEN Yanfeng, et al. Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in maize [J]. DNA Res, 2012, 19: 153 − 164. [20] MENG Dong, LI Yuanyuan, BAI Yang, et al. Genome-wide identification and characterization of WRKY transcriptional factor family in apple and analysis of their responses to waterlogging and drought stress [J]. Plant Physiol Biochem, 2016, 103: 71 − 83. [21] ROSS C A, LIU Yue, SHEN Qingxi J. The WRKY gene family in rice (Oryza sativa) [J]. J Integr Plant Biol, 2007, 49(6): 827 − 836. [22] SUN Chuanxin, SARA P, OLSSON H, et al. A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter [J]. Plant Cell, 2003, 15(9): 2076 − 2092. [23] 罗昌国, 渠慎春, 张计育, 等. 湖北海棠MhWRKY40b 在几种胁迫下的表达分析[J]. 园艺学报, 2013, 40(1): 1 − 9. LUO Changguo, QU Shenchun, ZHANG Jiyu, et al. Expression analysis of Malus hupehensis (Pamp) Rehd. MhWRKY40b gene in response to several stresses [J]. Acta Hortic Sin, 2013, 40(1): 1 − 9. [24] ZHANG Ying, YU Hongjun, YANG Xueyong, et al. CsWRKY46, a WRKY transcription factor from cucumber, confers cold resistance in transgenic-plant by regulating a set of cold-stress responsive genes in an ABA-dependent manner [J]. Plant Physiol Biochem, 2016, 108: 478 − 487. [25] ZHU Hong, ZHOU Yuanyuan, ZHAI Hong, et al. A novel sweetpotato WRKY transcription factor, IbWRKY2, positively regulates drought and salt tolerance in transgenic Arabidopsis [J]. Biomolecules, 2020, 10: 506. doi: 10.3390/biom10040506. [26] 王官凤, 吕兵兵, 王安虎, 等. 苦荞抗旱相关转录因子基因FtWRKY10的克隆及功能鉴定[J]. 农业生物技术学报, 2020, 28(4): 629 − 644. WANG Guanfeng, LÜ Bingbing, WANG Anhu, et al. Cloning and functional identification of drought resistance related transcription factor gene FtWRKY10 from tartary buckwheat (Fagopyrum tataricum) [J]. J Agric Biotechnol, 2020, 28(4): 629 − 644. [27] ZHANG Youzhi, LI Yaping, HASSAN M J, et al. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes [J]. BMC Plant Biol, 2020, 20: 150. doi: 10.1186/s12870-020-02354-y. [28] JIANG Wenbo, YU Diqiu. Arabidopsis WRKY2 transcription factor mediates seed germination and postgermination arrest of development by abscisic acid [J]. BMC Plant Biol, 2009, 9: 96. doi: 10.1186/1471-2229-9-96. -

-
链接本文:
https://zlxb.zafu.edu.cn/article/doi/10.11833/j.issn.2095-0756.20200706






 下载:
下载: