-
秀珍菇Pleurotus geesteranus是一种常见的食用菌[1],营养丰富、味道鲜美。但秀珍菇采后易发生病害而造成品质下降,常温下仅能储藏2~3 d[2]。微生物侵染是秀珍菇储藏过程中品质下降的主要原因。秀珍菇由于质地脆嫩,品质易受温度影响,传统低温储藏虽能在一定程度上延长秀珍菇保鲜期,但易产生低温冷害,无法长期保存[3-4]。食用菌采后病害影响其储藏品质,不同种类食用菌的致病菌不同,哈茨木霉Trichoderma harzianum是引发金针菇绿霉病的主要致病菌,托拉斯假单胞杆菌Pseudomonas tolaasii能够引发双孢菇湿泡病等[4]。
植物精油是一类从植物中提取的次生代谢物质,具有广谱的抑菌性,易于生物降解和相对安全的特性,近年来在果蔬采后病害防治领域应用广泛[5-6],但在食用真菌的防腐保鲜研究较少。具有采后病害控制作用的植物精油主要有香茅Mosla chinensis精油、桉Eucalyptus robusta精油、甜橙Citrus sinensis精油等,在果蔬的防腐保鲜中均有较好的防治效果[7-9],精油是具有潜在应用价值的生物防治保鲜剂[10-12],不同的精油对各类果蔬采后致病菌的防治效果不尽相同。本研究对秀珍菇采后储藏中的主要病原菌进行分离鉴定,筛选有效精油对其进行抑制,以期为植物精油的秀珍菇采后保鲜提供理论依据。
-
秀珍菇采自浙江杭州临安鼎新农业科技有限公司;精油购于江西国光香料厂(纯度>99%),主要来自檀香Santalum album、艾蒿Artemisia argyi、石菖蒲Acorus tatarinowii、甜橙、香茅、洋甘菊Anthemis nobilis、青蒿Artemisia annua、柏木Cupressus funebris、大蒜Allium sativum、橘Citrus reticulata、花椒Zanthoxylum bungeanum、茴香Foeniculum vulgare、桉等植物。
培养基有马铃薯葡萄糖琼脂培养基(PDA,上海博微生物科技有限公司)和马铃薯葡萄糖肉汤培养基(PDB,上海博微生物科技有限公司)。
-
主要仪器有LDZX50KBS立式压力蒸汽灭菌锅(上海申安医疗器械)、智能光照培养箱(宁波海曙赛福实验仪器厂)、SW-CJ-IC型双人单面净化工作台(苏州苏信环境科技有限公司)、HI2315型高精度电导率仪(北京哈纳科科技有限公司)、Sigma 500扫描电子显微镜(德国)、HZ-2111KA恒温摇床(华利达实验设备公司)。
-
用无菌手术刀在秀珍菇发病处切取病害和健康交界处的组织,用体积分数为75%乙醇表面消毒30 s,无菌水冲洗3次,无菌滤纸吸干水分后置于PDA平板中心,28 ℃培养3~5 d至组织发病。用无菌接种针挑取病症表面菌丝,置于PDA平板中心,28 ℃培养5 d后,挑取边缘菌丝纯化接种,直至菌落单一。出现单一菌落后,接入PDA斜面试管,用甘油封存后置于4 ℃冰箱保存。
-
根据柯赫氏法则,确定秀珍菇致病性。挑取健康无病害,无机械损伤,大小均一的秀珍菇,采用针刺法将纯化后的菌丝回接至秀珍菇表面。置于室温(25 ℃)培养,观察发病情况,以不接菌丝作为对照。发病后进行致病菌的分离纯化,方法同1.3.1。判断发病后纯化的菌株与回接的菌株是否一致,如一致,则进行下一步试验。
-
将试管中保存的菌丝活化,接种于PDA平板中,于28 ℃下培养,每天观察菌落特征,产孢后在菌落边缘挑取适量菌丝,在光学显微镜下观察菌丝及孢子形态,并根据其形态学特征初步判断其种属。
-
将培养4~5 d的平板,委托上海生工生物技术有限公司进行序列测定。内部转录间隔区(ITS)序列扩增引物为ITS1和ITS4[13],其引物序列为ITS1: 5′-TCCGTAGGTGAACCTGCGGC-3′;ITS4: 5′-TCCTCCGCTTATTGATATGC-3′,测序所得rDNA序列在美国国家生物技术信息中心(NCBI)网站上,利用BLAST将ITS序列与数据库中的序列进行对比,寻找同源性较大的物种,下载其序列,利用MEGA软件构建该菌株的系统发育树,并根据其序列的相似度,确定其所属物种。
-
将不同种类的精油配制成体积分数为500 μL·L−1的PDA平板,用无菌打孔器在病原菌平板上打取直径6 mm的菌饼,放入平板中央,28 ℃培养至6 d时,用游标卡尺测量菌落直径并记录[14-15]。设置3次重复。
-
用无菌打孔器在病原菌平板上打取直径6 mm 的菌饼,放入PDA平板中央,28 ℃培养,定期观察菌落,用游标卡尺测量菌落直径并记录[16-17],设置3次重复。
-
将孢子悬浮液分别混合于体积分数为0 (ck)、250、500、750、1 000 μL·L−1的石菖蒲精油和质量浓度为1.0%的葡萄糖混合溶液中,将上述混合溶液50 µL分别滴入双凹载玻片,置于培养皿中(直径 200 mm),25 ℃保湿培养,6、12、18 h时在显微镜下观察孢子萌发情况,每个处理镜检孢子数为200 个,以芽管长度超过孢子直径一半计为孢子萌发[18],设置3次重复。孢子萌发率计算公式为:孢子萌发率=(萌发孢子数/检查孢子总数)×100%
-
①丙二醛(MDA)质量摩尔浓度测定。将体积分数为0.1%的吐温80与石菖蒲精油混合充分后乳化,在PDB中加入一定量的孢子悬浮液,使孢子终浓度为109 CFU·L−1。分别抽滤取5 g菌丝在体积分数为0 (ck)、250、500、750、1 000 μL·L−1石菖蒲精油的PBS缓冲液中培养,0、2、4、6、8、10 h后取0.2 g菌丝冰浴研磨,加入1 mL无菌双蒸水提取,4 ℃ 10 000 r·min−1,20 min离心取上清液,采用硫代巴比妥酸法测定吸光度。②相对电导率测定。抽滤取5 g PDB中的菌丝在不同体积分数(0、250、500、750、1 000 μL·L−1)的石菖蒲精油-PBS缓冲液中培养,分别在0、10、20、40、60、120、180 min取样测定电导率,并计算相对电导率[19],设置3次重复。
-
将菌丝(湿质量2 g)加入到体积分数为0 (ck)、250、500、750、1 000 μL·L−1的石菖蒲精油无菌水中,28 ℃下摇床培养,分别于1、2、3、4、5 h后收集上清液测量可溶性碳水化合物、可溶性蛋白和核酸泄漏量[20],设置3次重复。以蔗糖为标准,使用蒽酮试剂法测量藤仓镰刀菌可溶性碳水化合物的泄漏程度;使用蛋白质检测试剂盒(生工生物技术有限公司,上海)检测可溶性蛋白泄漏程度;通过检测上清液在波长260 nm处的吸光度D(260)来判断核酸的泄漏程度,设置3次重复。
-
菌丝固定采用戊二醛、锇酸的双重固定法。取适量0、500 μL·L−1的石菖蒲精油平板上生长的菌丝边缘部分,浸泡于体积分数为3%的戊二醛溶液中,20 ℃固定,过夜后用pH 6.8的PBS缓冲液冲洗15 min,而后用体积分数为1%的锇酸溶液4 ℃固定2 h,再经乙醇梯度脱水、包埋、聚合、切片,经醋酸双氧铀和柠檬酸铅染色后置于透射电镜下观察[21]。
-
采用GraphPad Prism 8进行绘图,通过SPSS 24.0单因素方差分析进行数据统计及差异显著性分析,显著性水平为0.05。
-
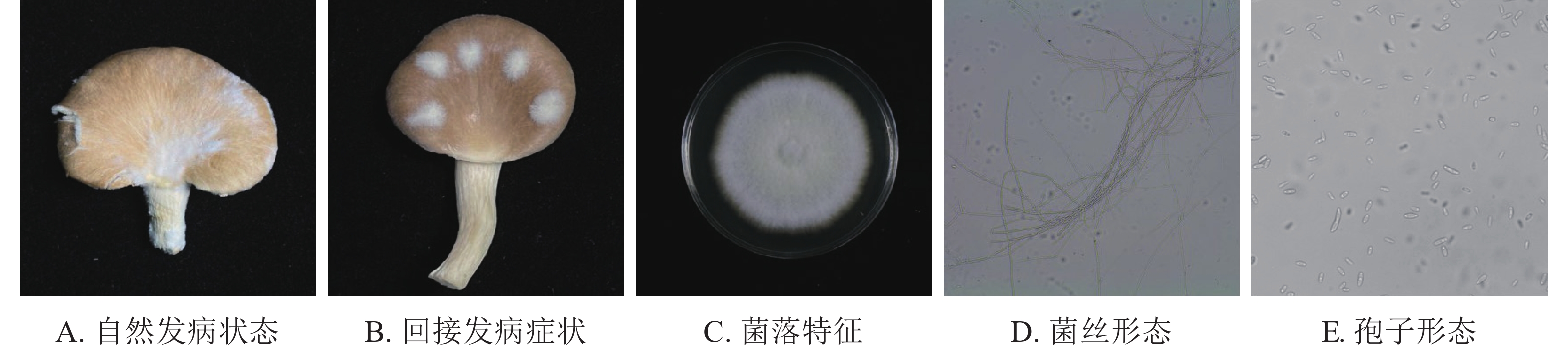
如图1A所示:在秀珍菇20 ℃储藏2 d出现病症后,根据组织分离法分离致病菌,按照不同的菌落形态和生长特征,分离到3株致病菌。
根据科赫氏法则,将分离的3株真菌回接至经过表面灭菌的秀珍菇上进行验证,发现仅1株菌株出现了与病症相似的表现(图1B)。再取回接发病病症处的菌丝进行纯化培养,观察到与其形态一致,证明该菌株为秀珍菇主要致病菌。
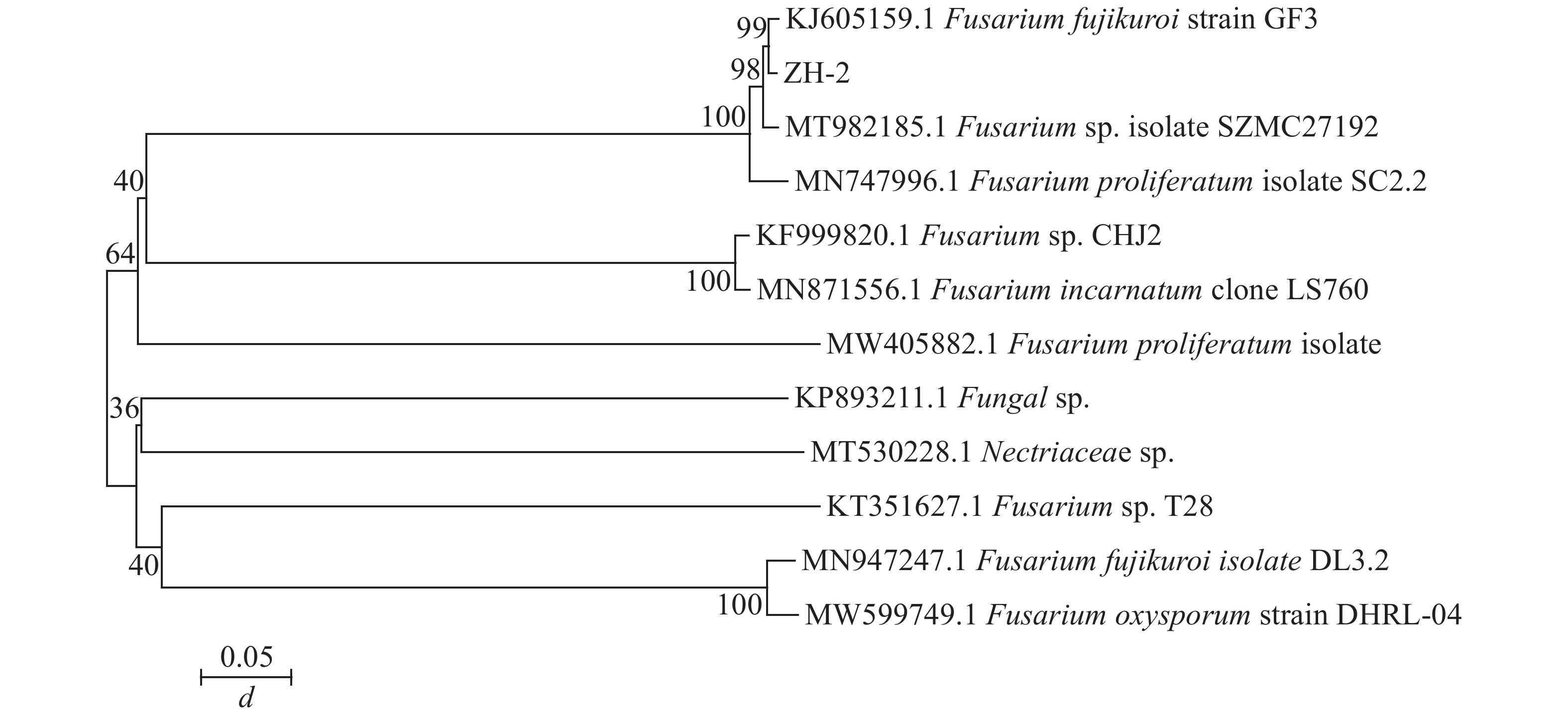
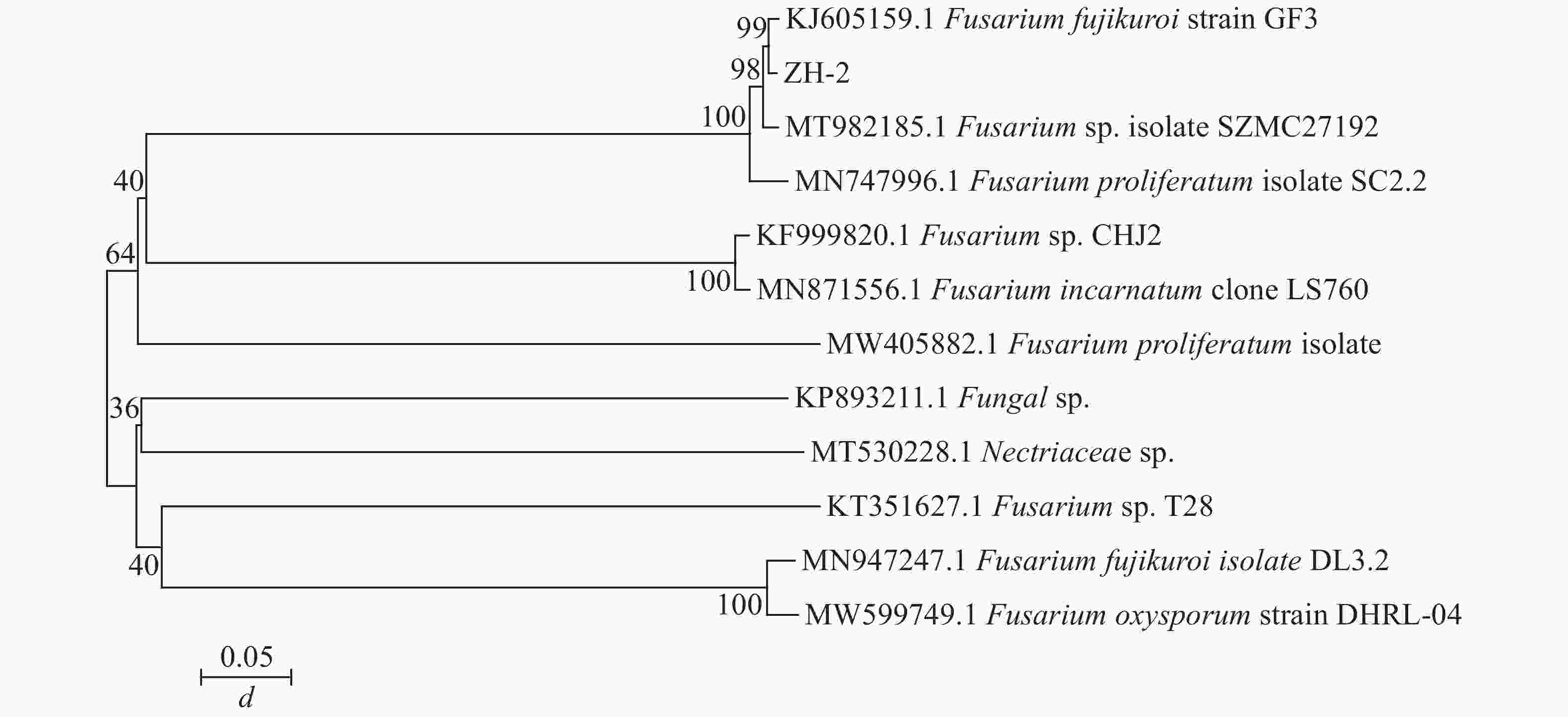
由图1C~E可见:菌落规则,圆形,棉絮状或毛毡状,菌丝体初期白色,生长旺盛,后期成淡黄色,菌丝有隔,分枝,大型分生孢子呈镰刀形或纺锤形,有隔膜,呈无色或透明。与镰刀菌属形态学基本一致。将测序所得的序列,利用NCBI网站的BLAST在线对比,将ITS序列与GenBank数据库中的核苷酸序列进行对比分析(图2),发现ITS序列与KJ605159.1 Fusarium fujikuroi strain GF3、MT982185.1 镰刀菌属Fusarium sp. isolate SZMC27192、MN747996.1 层生镰刀菌Fusarium proliferatumi isolate SC2.2、KF999820.1 Fusarium sp. CHJ2、MN871556.1 甜瓜镰刀菌Fusarium incarnatum clone LS760、MW405882.1 Fusarium proliferatumi isolate、KP893211.1 Fungal sp.、MT530228.1丛赤壳属Nectriaceae sp.、KT351627.1 Fusarium sp. T28、MN947247.1 Fusarium fujikuroi isolate DL3.2、MW599749.1 尖孢镰刀菌Fusarium oxysporum strain DHRL-04有不同程度的相似,对比发现该菌株与KJ1605159.1 Fusarium fujiuroi strain GF3同源性达99%,基本确定该菌株为藤仓镰刀菌。另外,选取几株同源性不同的序列,下载序列利用Neighbor-Joining方法构建系统发育树。由图2可知:该菌株与藤仓镰刀菌聚为一支,有非常近的亲缘关系。结合形态学特征与生物学鉴定结果分析,确定该致病菌为藤仓镰刀菌。
-
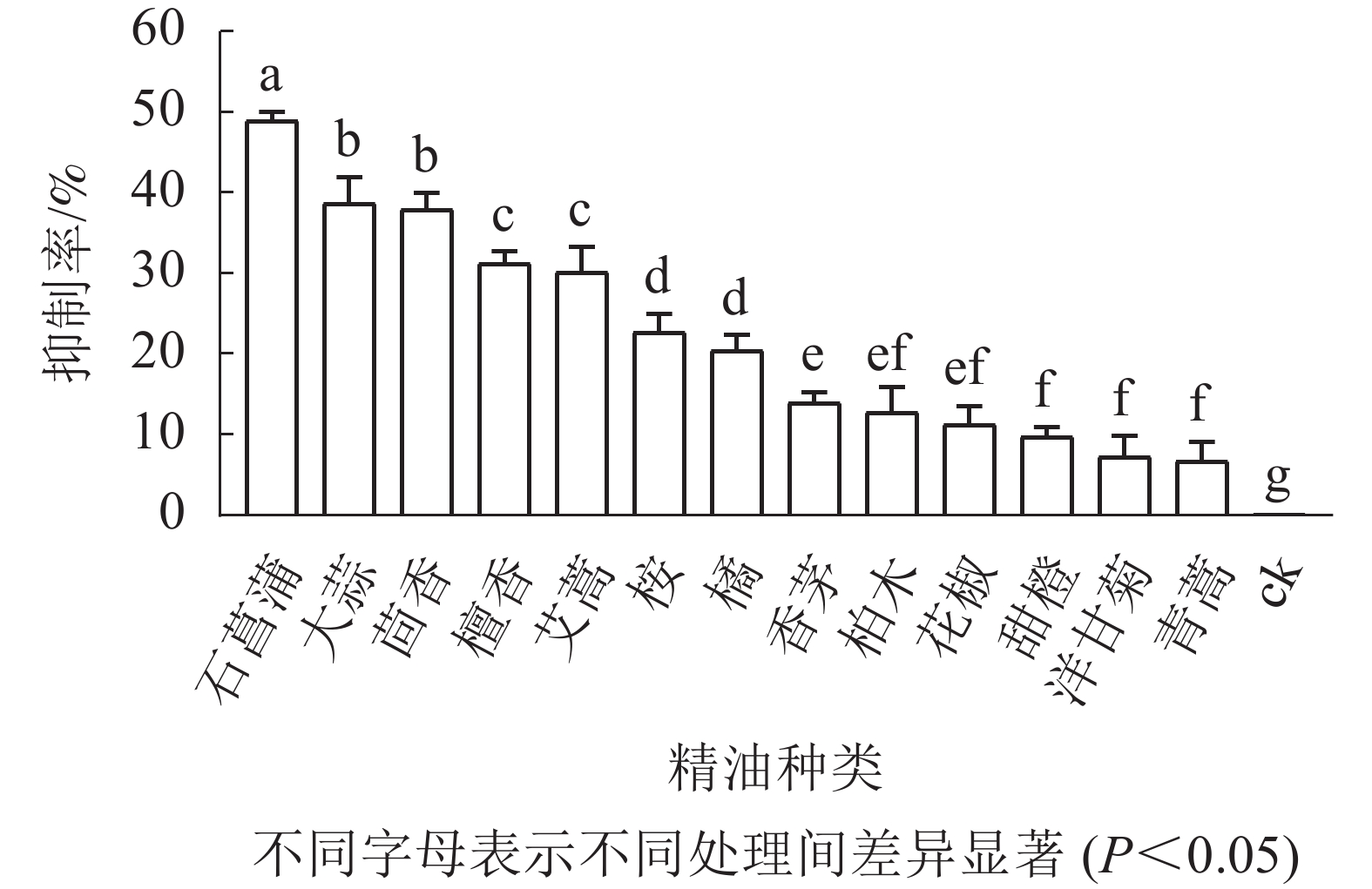
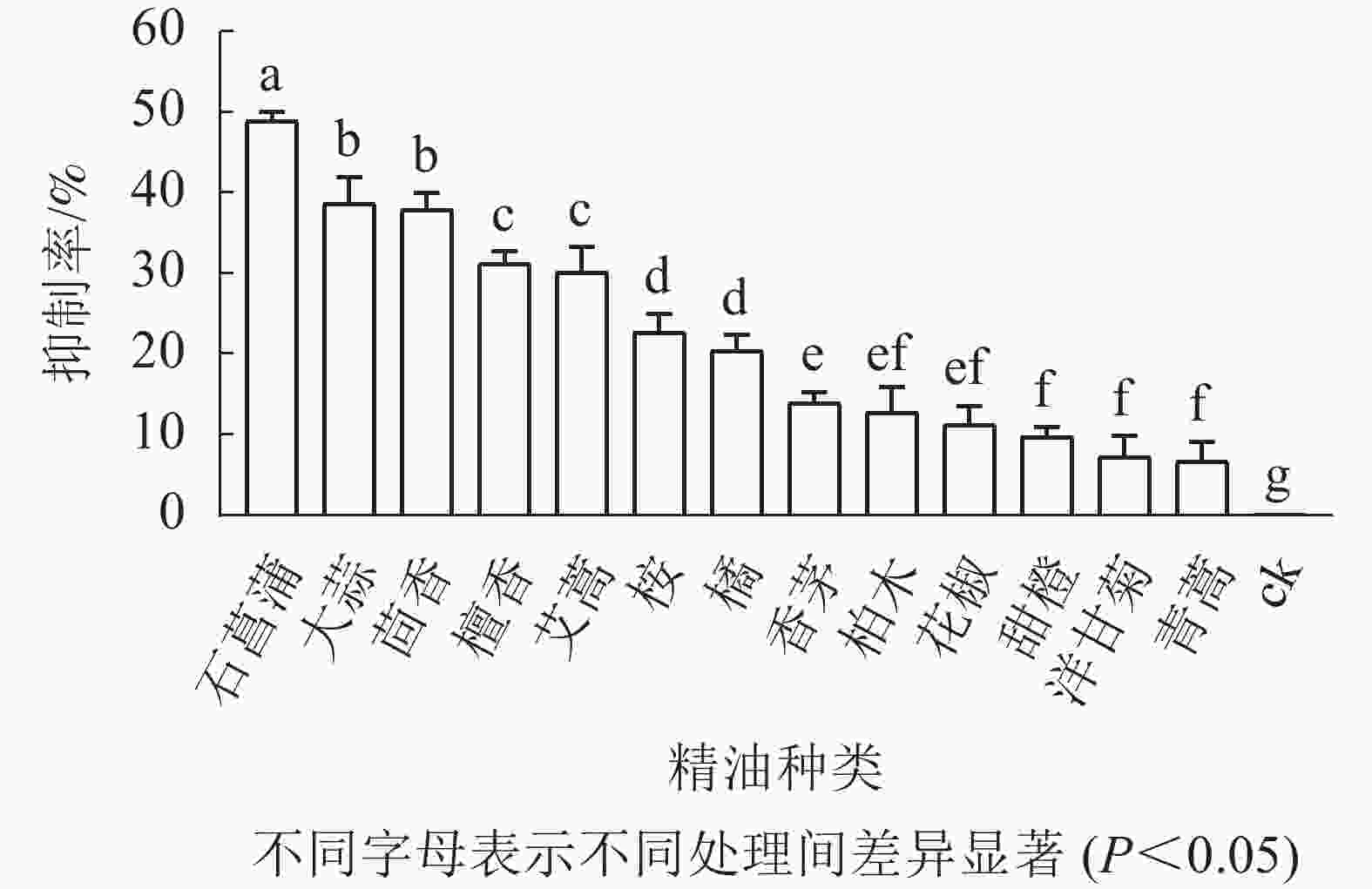
如图3所示:培养5 d后,藤仓镰刀菌在不同精油平板上的菌落直径不同,菌落直径越小表征菌丝生长越缓慢,则该精油的抑制效果越好。石菖蒲精油在13种精油中对藤仓镰刀菌的抑制效果最佳,故选择石菖蒲精油进一步研究。
-
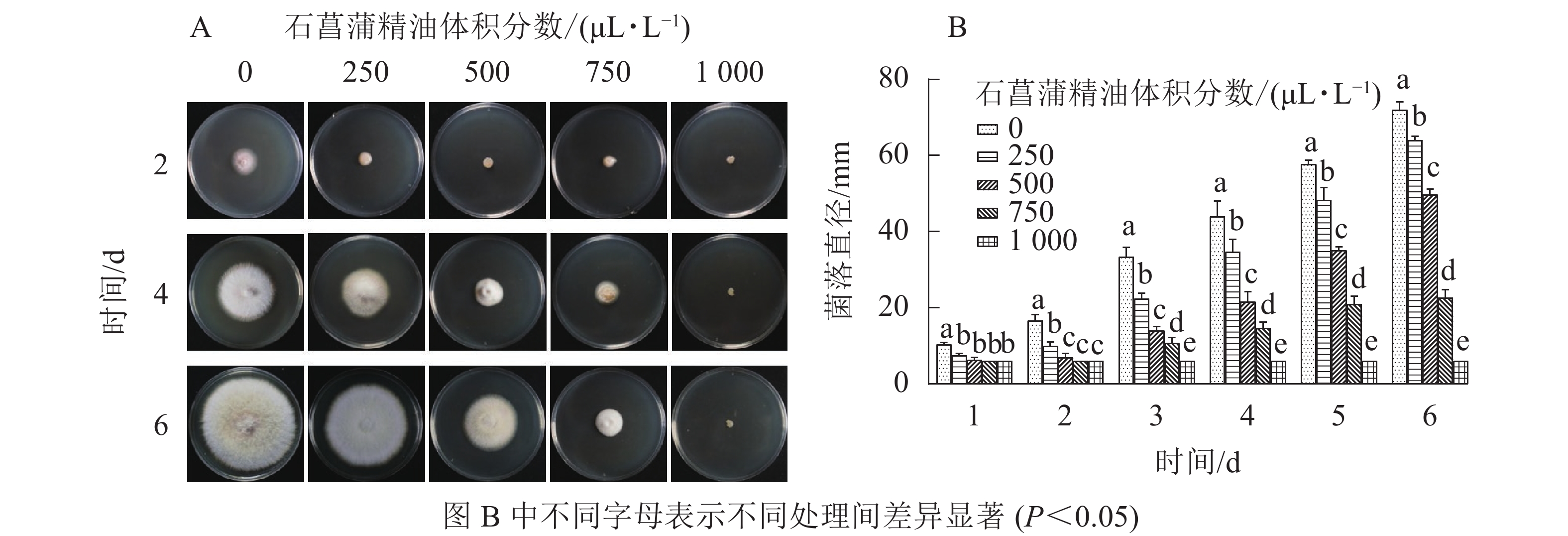
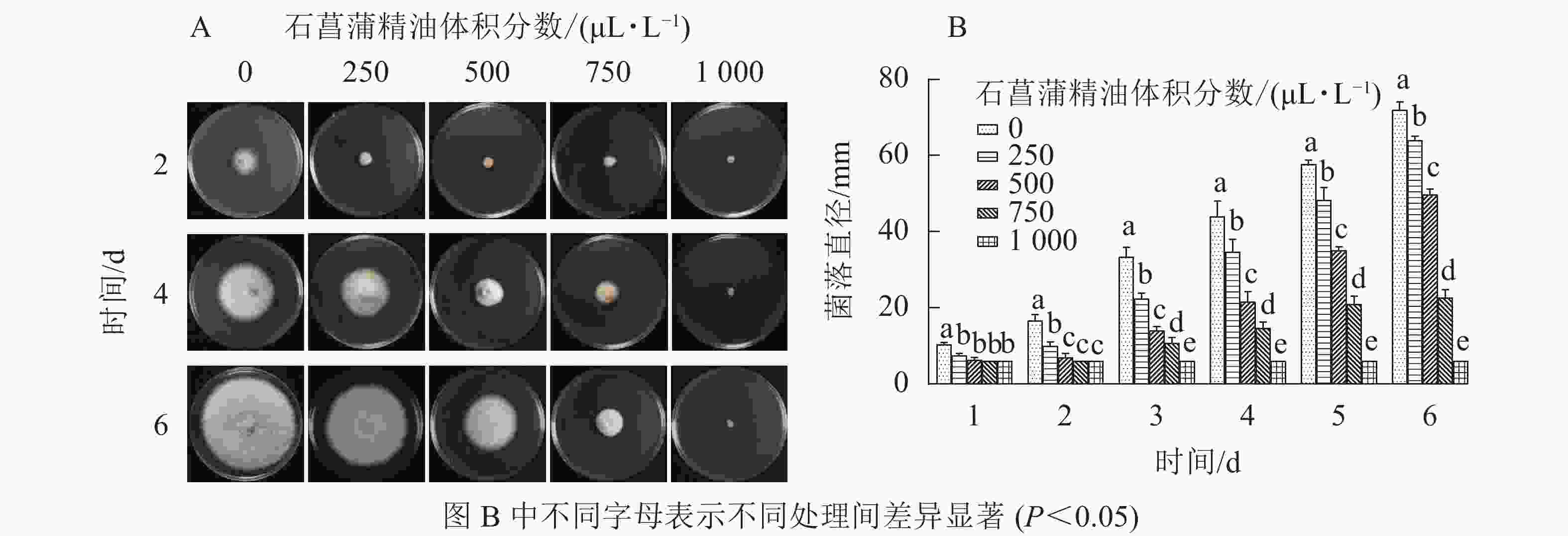
由图4可知:250 μL·L−1的石菖蒲精油对藤仓镰刀菌菌丝生长有明显的抑制效果,培养4 d后不同体积分数处理的菌丝生长存在显著差异(P<0.05),1 000 μL·L−1的石菖蒲精油能完全抑制藤仓镰刀菌的生长,且转接至无精油PDA平板后依旧不生长。可确定石菖蒲精油对藤仓镰刀菌的半数效应体积分数(抑菌率达50%的体积分数为半数效应体积分数,EC50)为500 μL·L−1,最小抑菌体积分数为1 000 μL·L−1。
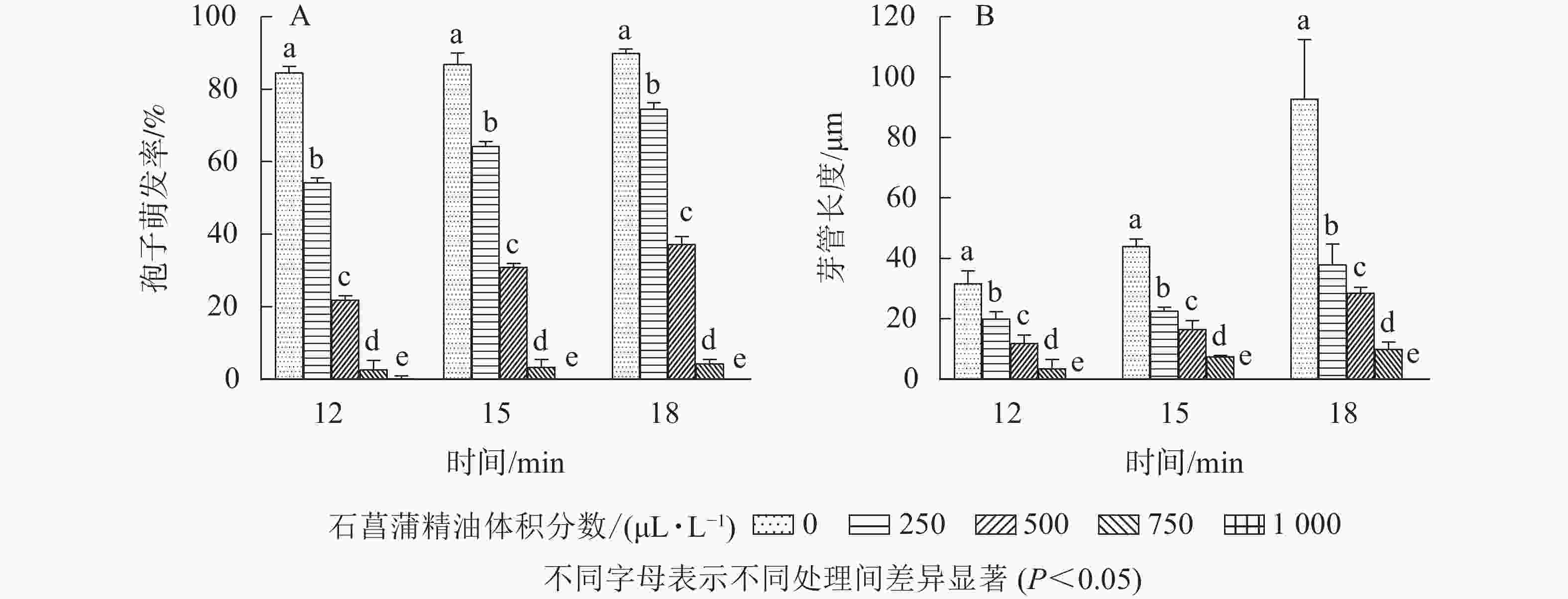
如图5所示:随着石菖蒲精油体积分数的升高,藤仓镰刀菌孢子萌发率显著降低(P<0.05)。处理18 h以后,对照组孢子萌发率达85.7%,而750和1 000 μL·L−1石菖蒲精油处理组孢子萌发率分别为4.2%和0,孢子萌发抑制率分别为95.1%和100.0%。表明在一定体积分数范围内,石菖蒲精油能显著抑制藤仓镰刀菌孢子的萌发。
-
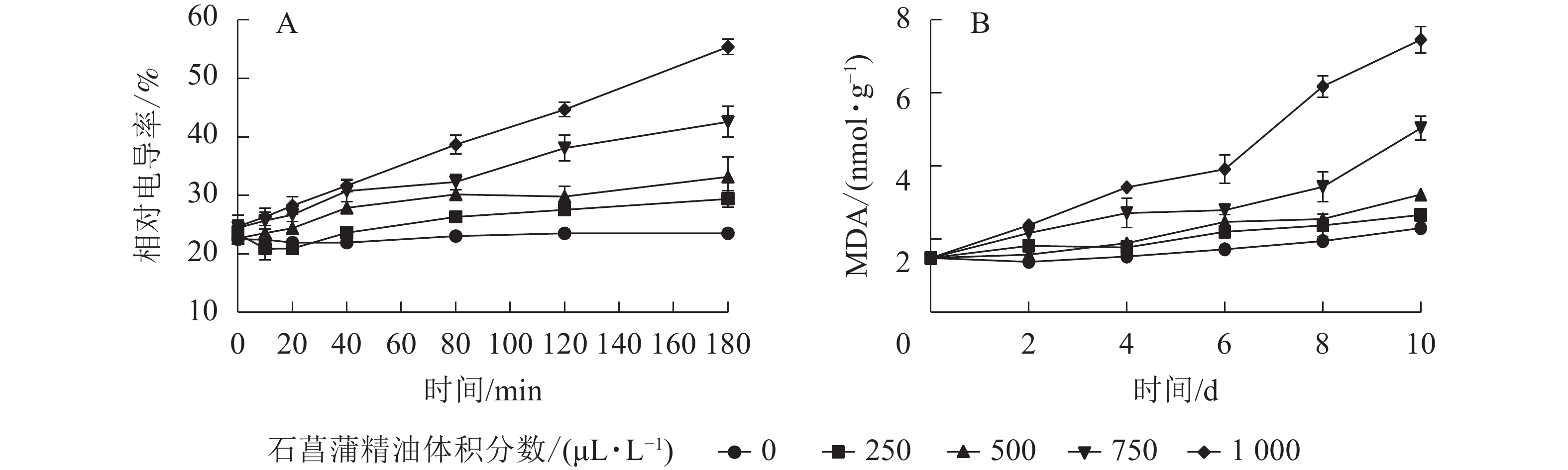
用相对电导率来表征膜通透率,相对电导率越高,膜通透性越大。如图6A所示:藤仓镰刀菌相对电导率随着石菖蒲精油体积分数的升高而升高。处理组在80 min后显著高于对照组(P<0.05)。3 h后对照组相对电导率为23.2%,而1 000 μL·L−1石菖蒲精油处理组的相对电导率达55.1%。表明精油处理可显著提高藤仓镰刀菌的相对电导率(P<0.05),从而可进一步增加高藤仓镰刀菌的膜通透性。
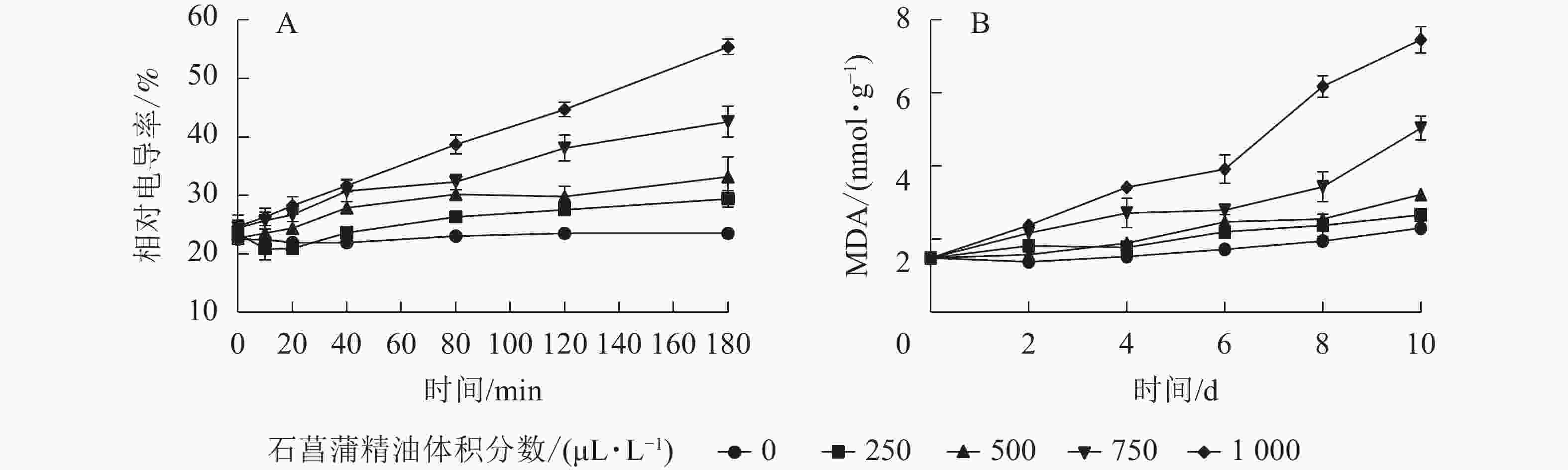
Figure 6. Effects of A. tatarinowii essential oil on F. fujikuroi Relative conductivity and MDA content
MDA是细胞膜脂过氧化的产物,可以反映细胞膜脂过氧化的程度,是指示膜系统受伤的重要指标之一。如图6B所示:经过石菖蒲精油处理的藤仓镰刀菌,MDA质量摩尔浓度显著高于对照,1 000 μL·L−1石菖蒲精油处理组在10 h后MDA质量摩尔浓度是对照的3倍。表明石菖蒲精油能够破坏细胞脂膜结构,从而导致MDA质量摩尔浓度上升,加速菌丝细胞的膜脂过氧化。
-
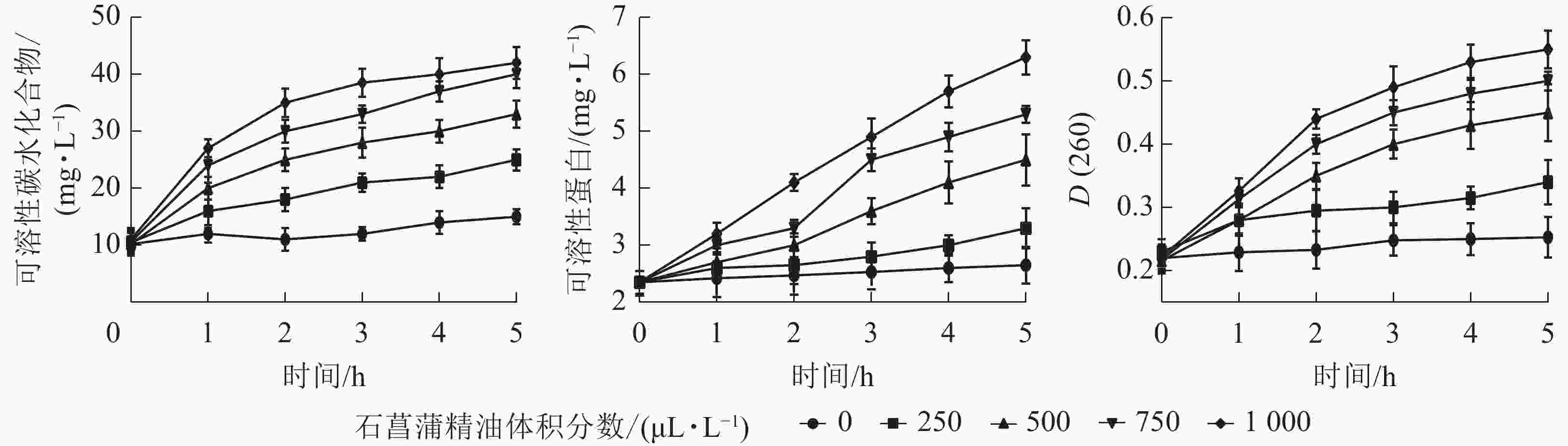
细胞内容物出现在培养液中说明细胞的质膜受到了破坏,也是细胞膜损伤的重要表征指标。如图7所示:培养液中可溶性蛋白、核酸、可溶性碳水化合物随着精油处理时间的延长都有不同程度的上升。表明经石菖蒲精油处理后,细胞内容物出现不同程度泄漏,且随着精油体积分数的增大而升高。说明精油处理能够破坏脂膜结构,从而导致细胞内容物泄漏。
-
采用扫描电镜观察石菖蒲精油对藤仓镰刀菌菌丝结构的影响,发现未经精油处理的菌丝表面光滑,存在孢子丛(图8A)。精油处理后(图8B)的菌丝皱缩,无孢子产生。进一步说明石菖蒲精油能够破坏藤仓镰刀菌菌丝结构,影响菌丝正常生长,并抑制孢子的产生。本研究结果与顾可飞等[13]发现精油可造成真菌菌丝畸形或使真菌菌丝断裂的结论基本一致。
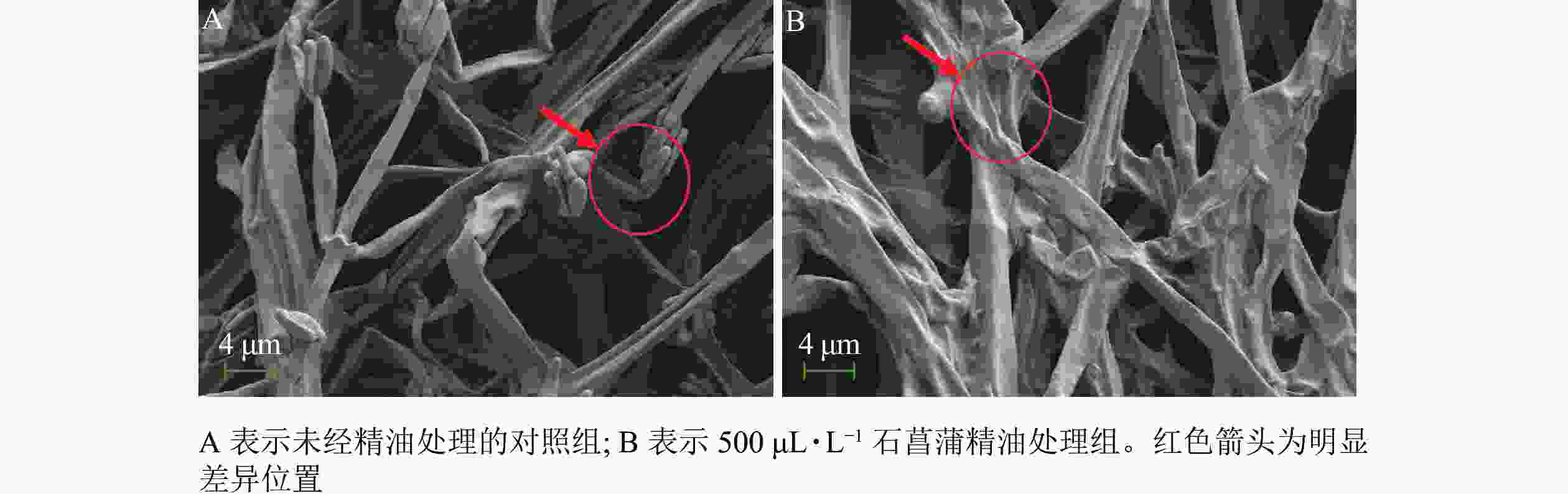
Figure 8. Sem observation of the effect of A. tatarinowii essential oil on F. fujikuroi mycelium structure
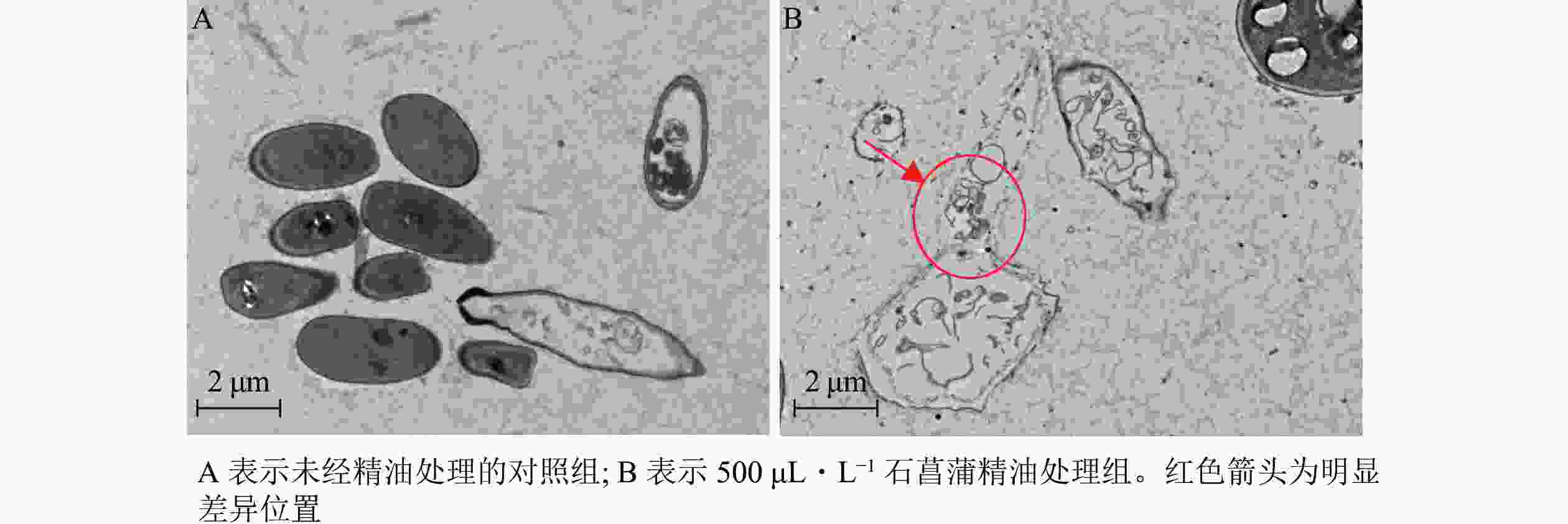
进一步采用透射电镜观察石菖蒲精油对藤仓镰刀菌细胞超微结构的影响,发现未经精油处理的细胞形态完整,质膜均匀,细胞壁清晰(图9A)。经石菖蒲精油处理的菌丝细胞膜和核膜破坏严重,细胞各结构也有较大破坏(图9B)。表明石菖蒲精油的抑菌机制可能是通过破坏藤仓镰刀菌细胞膜的完整性,暴露和侵蚀细胞器,造成细胞损伤,最终导致细胞死亡。
-
本研究对秀珍菇的采后致病菌进行分离,根据科赫氏法则确定其致病性,在形态上致病菌菌落呈白色,后期为淡黄色,菌丝有隔有分支,孢子呈镰刀型,在形态学上对比发现其与镰刀菌形态学描述一致;利用rDNA-ITS序列对比和系统发育树分析的方法,发现致病菌与藤仓镰刀菌相似度达99%,由此确定藤仓镰刀菌为秀珍菇采后储藏中的主要致病真菌,该菌在百香果Passiflora eduli[22]、樱桃番茄Lycopersicon esculentum var. cerasiforme[23]等农产品均见报道,是较为常见的致病菌。
研究13种常见植物精油对对藤仓镰刀菌抑制效果时发现:石菖蒲精油对藤仓镰刀菌控制效果最佳,其EC50为500 μL·L−1,最小抑菌体积分数为1 000 μL·L−1。相同体积分数处理下,石菖蒲精油对孢子萌发的抑制率高于对菌丝生长的抑制率,说明藤仓镰刀菌孢子对石菖蒲精油的敏感度更高,这与PEI等[24]探究香芹酚对炭疽菌的抑制效果相似。
经对藤仓镰刀菌培养液中MDA和相对电导率变化情况的观测,发现石菖蒲精油的处理可使藤仓镰刀菌的胞内MDA质量摩尔浓度增加,MDA质量摩尔浓度和相对电导率是表征菌丝细胞膜脂过氧化程度和细胞膜结构破坏程度的重要指标[25],MDA质量摩尔浓度增加表明石菖蒲精油能够加速藤仓镰刀菌的膜脂氧化,加剧细胞内物质的外泄;而扫描电镜和透射电镜结果也显示藤仓镰刀菌菌丝表面出现褶皱,菌丝细胞壁膜被破坏。LIU等[26]研究表明:香草精油的抗真菌活性是由于其能够破坏细胞膜结构。PEI等[24]发现:香芹酚能够使炭疽菌细胞膜通透性增加。本研究表明:石菖蒲精油能够有效抑制藤仓镰刀菌作用机制,破坏藤仓镰刀菌膜系统结构,引起内容物泄漏,进而导致菌体死亡。
Identification of postharvest pathogenic bacteria and the bacteriostasis of plant essential oil for Pleurotus geesteranus
doi: 10.11833/j.issn.2095-0756.20210819
- Received Date: 2021-12-28
- Accepted Date: 2022-05-26
- Rev Recd Date: 2022-05-06
- Available Online: 2023-06-05
- Publish Date: 2022-12-20
-
Key words:
- Pleurotus geesteranus /
- postharvest disease control /
- Fusarium fujiuroi /
- Acorus tatarinowii essential oil /
- bacteriostat
Abstract:
| Citation: | ZHANG Yi, CUI Jie, HE Ruoming, et al. Identification of postharvest pathogenic bacteria and the bacteriostasis of plant essential oil for Pleurotus geesteranus[J]. Journal of Zhejiang A&F University, 2022, 39(6): 1321-1329. DOI: 10.11833/j.issn.2095-0756.20210819 |









 DownLoad:
DownLoad: