-
枸杞Lycium为茄科Solanaceae枸杞属Lycium多年生落叶灌木,具有很强的抗逆性,是改良盐碱地的先锋树种[1]。全世界约有80种,主要分布在北美洲和亚洲[2]。中国自然分布有7种3变种,多分布于西北和华北地区。在诸多种中,绝大多数种质资源未被利用,只有宁夏枸杞Lycium barbarum经过长期自然选择、人工驯化,形成了10多个农家品种[1],其干燥果实具有味甘、性平、归肝、肾经、益精、明目的功能,被历版《中国药典》收载[3]。宁夏农林科学院自20世纪80年代开始枸杞种质资源收集与保存工作,目前,已建成世界上唯一枸杞种质资源圃,保存枸杞种质2 000余份[4]。枸杞属于浆果类植物,其果实中含有丰富糖类物质[5]。SUNG等[6]通过气相色谱-质谱联用仪(GC-MS)在宁夏枸杞果实中检出11种单糖;杨晓萍等[7]通过气相色谱(GC)测定宁夏枸杞果实单糖的种类,主要是果糖、葡萄糖和木糖;欧阳华学等[8]利用高效液相色谱法测定枸杞中单糖和低聚糖,主要是鼠李糖、果糖、葡萄糖等3种单糖和蔗糖、麦芽糖2种低聚糖;冯美等[9]发现枸杞果实中糖的积累主要以葡萄糖和果糖为主;郑国琦等[10]利用高效液相色谱测定不同地区宁夏枸杞果实糖质量分数,果实内的果糖量最高,葡萄糖量其次,蔗糖量最低。蔗糖是植物体内最重要的一种碳水化合物,蔗糖代谢起主要作用的酶有蔗糖合成酶(SS)、磷酸蔗糖合成酶(SPS)和蔗糖转化酶(IN),它们在糖代谢中发挥着重要的作用[11]。目前,枸杞蔗糖代谢的相关研究主要集中在宁夏枸杞[12-15]上,其他种枸杞资源尚未报道。本研究选用4种枸杞为试材,研究枸杞果实发育过程中糖质量分数与蔗糖代谢酶活性的关系,旨在探讨枸杞果实糖积累差异的生理基础,为进一步阐明枸杞品质形成和调控机制提供理论依据。
HTML
-
试验材料分别为宁夏枸杞Lycium barbarum,北方枸杞L. chinense var. potaninii,云南枸杞L. yunnanense和中国枸杞L. chinense,均来自于宁夏农林科学院西夏区芦花台园林试验场枸杞种质资源圃,10~15年生枸杞植株。选无性系2株·材料-1,选取果实发育的幼果期(开花后9~12 d),青果期(开花后14~19 d),色变期(开花后20~25 d),初熟期(开花后25~30 d)和成熟期(开花后34~45 d)等5个时期进行采样。4种枸杞形态特征见图 1。样品采集后用铝箔纸包装放到液氮速冻,运送回实验室立即放置到超低温冰箱中保存。
-
采用气相色谱(GC)进行糖质量分数测定。称取枸杞鲜样3.0 g,置于研钵中研磨后,立即倒入鸡心瓶中,加体积分数为80%乙醇溶液75.0 mL,回流提取1 h,趁热过滤,残渣用体积分数为80%热乙醇溶液洗涤(约25.0 mL),洗后溶液一同过滤并入滤液中,冷却后定容至100.0 mL。移取4.0 mL滤液至具塞玻璃管中,抽气泵将其抽干,加入吡啶1.0 mL摇动使其溶解后,于冰水浴中依次加入0.4 mL六甲基二硅胺烷、0.2 mL三甲基氯硅烷,于20 ℃条件下静置30 min,离心,收集上清液,用气相色谱(GC)测定。气相测定条件:升温程序为初温180 ℃,保持20 min,以20 ℃·min-1升至280 ℃,保持10 min;FID检测器温度为300 ℃;进样口温度为280 ℃;氦气(He)流速30.0 mL· min-1;氮气(N2)流速30.0 mL·min-1;空气流速300.0 mL·min-1;分流比为20∶1;进样量为1.0 μL。试验所需标样(果糖、葡萄糖、蔗糖、赤鲜糖、阿拉伯糖、鼠李糖、岩藻糖、半乳糖)均为Sigma公司生产。
-
酸性转化酶(AI)活性测定按照LOWELL等[16]方法进行,略有改动。在1.0 mL的反应液中含0.6 mL 0.1 mol·L-1乙酸钠-乙酸(pH 4.8),0.2 mL 0.1mol·L-1蔗糖,0.2 mL酶提液。在37 ℃水浴40 min后,加入1.0 mL 3,5-二硝基水杨酸试剂(DNS)充分反应,在沸水浴5 min,冷却至室温,4 000 r·min-1离心10 min,测定吸光度D(520)值,对照在反应体系中加入煮沸后酶液。用两者的差值来计算还原糖产生速率,表示转化酶的活性,通过测定还原糖的生成量表示其活性。中性转化酶(NI)活性测定方法同AI,只是把乙酸钠-乙酸(pH 4.8)换成柠檬酸钠-柠檬酸(pH 7.2)。磷酸蔗糖合成酶(SPS)活性测定,70.0 μL反应体系中含50.0 mmol·L-1 4-羟乙基哌嗪乙磺酸(hepes)-氢氧化钠(pH 7.5),15 mmol·L-1 氯化镁,1.0 mmol·L-1乙二胺四乙酸(EDTA),16.0 mmol·L-1尿苷二磷酸葡萄糖(UDPG),4.0 mmol·L-1 6-磷酸果糖(F-6-P),20.0 mmol·L-1 6-磷酸葡萄糖(G-6-P),30.0 μL酶提取液。30 ℃反应30 min,加入70.0 μL 5 mol·L-1 氢氧化钠终止反应,沸水浴10 min,冷却后加入1.0 mL 0.14 mol·L-1蒽酮(溶解在13.80 mol· L-1的硫酸中),40 ℃反应20 min,测定吸光度D(620)。对照的反应体系中不含6-磷酸果糖和6-磷酸葡萄糖。蔗糖合成酶(SS)活性测定与SPS类似,将反应体系中的6-磷酸果糖换成果糖。
-
利用Excel 2003和DPS数据处理系统(14.5高级版)进行数据统计,采用最小显著性差异(LSD)法进行显著性差异分析。
1.1. 试验材料
1.2. 试验方法
1.2.1. 糖质量分数测定
1.2.2. 蔗糖代谢酶活性测定
1.3. 数据处理
-
通过气相色谱(GC)法对4种枸杞成熟期果实糖组成和质量分数进行测定(表 1)。宁夏枸杞检测出10种糖,种类最多,分别为果糖、葡萄糖、蔗糖、赤藓糖、阿拉伯糖、鼠李糖、岩藻糖、半乳糖、木糖和山梨糖;云南枸杞和中国枸杞检测到7种糖,分别为果糖、葡萄糖、蔗糖、赤藓糖、阿拉伯糖、岩藻糖和半乳糖;北方枸杞检测到6种糖,分别为果糖、蔗糖、葡萄糖、赤藓糖、阿拉伯糖和半乳糖。在4种枸杞果实中均检测到糖为果糖、葡萄糖、蔗糖、赤藓糖、阿拉伯糖和半乳糖,其中,果糖、葡萄糖和蔗糖质量分数占总糖分的98%以上。宁夏枸杞果实果糖和葡萄糖质量分数最高,北方枸杞次之,云南枸杞和中国枸杞较低。云南枸杞果实蔗糖质量分数最高,北方枸杞次之,宁夏枸杞最低。4种枸杞间3种糖的质量分数均达到显著差异。可见,不同种枸杞的糖组成与质量分数存在较大差异。
糖组分 不同种枸杞的糖质量分数/(mg·g-1) 最小显著性差异 (LED,P<0.05) 宁夏枸杞 北方枸杞 云南枸杞 中国枸杞 果糖 48.99±1.081 31.57±1.112 9.39±0.300 14.99±0.221 3.874 葡萄糖 2.34±0.063 1.57±0.077 0.42±0.025 0.71±0.030 0.118 蔗糖 0.43±0.068 1.11±0.156 1.40±0.054 0.96±0.027 0.189 赤藓糖 0.07±0.003 0.16±0.003 0.31±0.025 0.03±0.001 0.027 阿拉伯糖 0.04±0.000 0.05±0.004 0.11±0.008 0.01±0.001 0.011 鼠李糖 0.02±0.000 岩藻糖 0.01±0.001 0.08±0.005 0.01±0.001 0.008 半乳糖 0.02±0.001 0.08±0.004 0.04±0.014 0.02±0.001 0.018 木糖 0.01±0.000 山梨糖 0.02±0.000 甜度值 87.79±2.000 57.46±2.160 18.14±0.600 27.69±0.380 6.900 Table 1. Changes of sugar contents in four wolfberry species at fruit ripening
分析不同种枸杞果实甜度值发现:宁夏枸杞甜度值最高,为87.79;北方枸杞次之,为31.57; 云南枸杞最低,仅为27.69。4种枸杞间甜度值均达到显著差异,这也说明宁夏枸杞口感显著优于其他3种枸杞(表 1)。
-
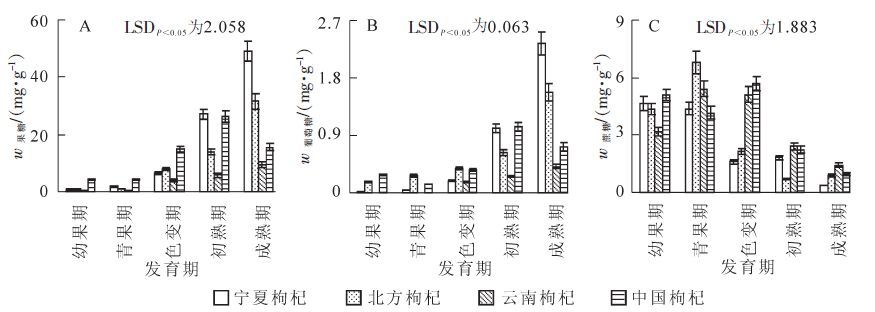
4种枸杞在果实发育过程中果糖和葡萄糖质量分数变化如图 2A和2B所示。随着果实的生长发育,果糖和葡萄糖质量分数总体呈现出不断升高趋势。从幼果期到青果期果糖和葡萄糖质量分数较低,呈现出缓慢增高趋势。在云南枸杞中未检测出葡萄糖,且中国枸杞2种糖质量分数显著高于其他3种枸杞;从青果期之后,宁夏枸杞、北方枸杞和中国枸杞呈现出显著增长,在初熟期,中国枸杞到达峰值,分别为26.09 mg·g-1和1.03 mg·g-1,而宁夏枸杞和北方枸杞仍缓慢增高,在果实成熟期达到最高值,此时,宁夏枸杞果糖和葡萄糖质量分数为49.08 mg·g-1和2.34 mg·g-1,北方枸杞为31.54 mg·g-1和1.58 mg·g-1,2种枸杞间达到显著差异。在整个果实发育过程中,云南枸杞维持着较低果糖和葡萄糖质量分数,且从初熟期到成熟期显著低于其他3种枸杞。
随着枸杞果实的生长发育,4种枸杞果实蔗糖质量分数呈现出2种变化趋势(图 2C),其中,宁夏枸杞和中国枸杞呈现出“降—升—降”趋势,从幼果期到色变期缓慢降低,在青果期中国枸杞出现第1个低谷值,之后缓慢升高,而宁夏枸杞继续不断降低,在色变期宁夏枸杞出现低谷值,而中国枸杞呈现出峰值,随后两者均呈现出快速降低,在果实成熟期到达最低值。北方枸杞和云南枸杞从幼果期到青果期缓慢升高,在青果期到达到最高,值分别为6.76 mg·g-1和5.40 mg·g-1,接着继续降低。在初熟期北方枸杞达到低谷值,而云南枸杞继续降低,且在整个发育过程中两者间均未达到显著差异。
-
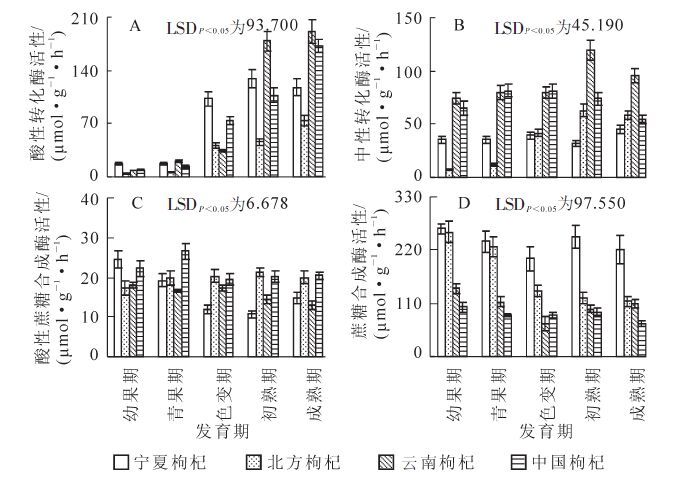
4种枸杞果实发育期过程中果实AI活性变化如图 3A所示,随着果实发育,AI活性呈现出2种变化趋势,宁夏枸杞呈现出先升后降的趋势,在果实发育的色变期之前AI活性增幅较小,没达到显著差异;随后快速升高,在果实发育的初熟期达到最高峰。然后又呈现出缓慢降低趋势。北方枸杞、云南枸杞和中国枸杞呈现出逐渐升高的趋势,在果实发育的色变期前缓慢升高,随后快速升高,成熟期达到最高值。在果实成熟期云南枸杞AI活性最高,达191.07 μmol·g-1·h-1,中国枸杞次之,北方枸杞最低,且显著低于云南枸杞和中国枸杞。在整个果实发育过程中北方枸杞维持较低AI活性。因此,可以看出从果实发育的幼果期到色变期,供试材料AI活性低、种间变化小,但从色变期到果实成熟,供试材料AI活性高、种间变化大。

Figure 3. Changes of sucrose-metabolizing enzymes activity in four wolfberry species at five stages of ripening
图 3B所示:从幼果期到色变期宁夏枸杞 NI活性缓慢升高,随后缓慢降低,接着又升高,在果实成熟期升至最高,为45.29 μmol·g-1·h-1;北方枸杞、云南枸杞和中国枸杞呈现出先升后降变化趋势,从幼果期到青果期不断升高,在色变期中国枸杞升至最高值,为81.07 μmol·g-1·h-1,而北方枸杞和云南枸杞继续升高,且在初熟期两者到达峰值,分别为62.50 μmol·g-1·h-1和119.46 μmol·g-1·h-1,两者间差异显著,随后缓慢降低。在整个果实发育过程,云南枸杞保持着较高NI活性,而宁夏枸杞和北方枸杞维持着较低NI活性。
4种枸杞果实发育期过程中果实SPS活性变化如图 3C所示,宁夏枸杞SPS活性呈现出先降后升的变化趋势,从幼果期到初熟期SPS活性缓慢降低,在初熟期低出现谷值,从初熟期到成熟期SPS活性缓慢升高;北方枸杞和中国枸杞从幼果期到青果期不断升高,在青果期中国枸杞升值最高值,达26.51 μmol·g-1·h-1,而北方枸杞继续升高,在初熟期达到最高值,为21.41 μmol·g-1·h-1,随后两者基本维持稳定水平;随着果实生长发育云南枸杞SPS活性总体呈现出降低趋势,从幼果期到色变期呈现出平稳状态,接着缓慢降低,在果实成熟降至最低,为12.95 μmol·g-1·h-1,显著低于北方枸杞和中国枸杞。在整个果实发育过程中,北方枸杞和中国枸杞保持着较高SPS活性,云南枸杞维持着较低SPS活性。可见,4种枸杞在果实发育过程中SPS活性差异较大。
4种枸杞果实发育期过程中果实SS活性变化(图 3D)表明:宁夏枸杞、云南枸杞和中国枸杞随着果实生长发育,从果实幼果期到色变期SS活性缓慢降低,从色变期到初熟期缓慢升高,在初熟期宁夏枸杞和中国枸杞达到峰值,而云南枸杞继续升高,且在整个果实发育过程中,宁夏枸杞SS活性显著高于后2种枸杞,后2种枸杞间无显著差异;北方枸杞SS活性呈现出不断降低趋势,从幼果期到青果期缓慢降低,随后显著下降,从初熟期到成熟期又缓慢下降,且此时期显著低于宁夏枸杞。
-
4种枸杞果实发育过程中糖质量分数与蔗糖代谢酶活性之间相关性分析表明:宁夏枸杞果糖质量分数与葡萄糖质量分数极显著正相关(r=0.993),与蔗糖质量分数显著负相关(r=- 0.830),与AI活性显著正相关(r=0.807),其蔗糖质量分数与AI活性显著负相关(r=-0.934);北方枸杞果糖质量分数与葡萄糖质量分数极显著正相关(r= 0.987),与AI和NI活性显著正相关(r=0.949和0.809),其蔗糖质量分数与AI和NI活性显著负相关(r=- 0.866和- 0.917),其蔗糖质量分数SS显著正相关(r=0.870);云南枸杞果糖质量分数与葡萄糖质量分数极显著正相关(r=0.999),与AI活性显著正相关(r=0.912),与SPS活性显著负相关(r=0.870),其葡萄糖质量分数与AI活性显著正相关(r=0.918),其葡萄糖质量分数与SPS活性显著负相关(r=- 0.891);中国枸杞果糖质量分数与葡萄糖质量分数显著正相关(r=0.925),其蔗糖质量分数与AI活性显著负相关(r=-0.806)(表 2)。可见,枸杞果实发育过程中降低蔗糖质量分数有利于果实中果糖和葡萄糖积累,转化酶在枸杞果实糖积累过程中发挥着重要作用。
材料 葡萄糖 蔗糖 酸性转化酶 中性转化酶 磷酸蔗糖合成酶 蔗糖合成酶 宁夏枸杞 果糖 0.993** -0.830* 0.807* 0.554 -0.500 -0.241 葡萄糖 -0.804 0.684 0.627 -0.423 -0.246 蔗糖 -0.934* -0.604 0.804 0.665 北方枸杞 果糖 0.987** -0.748 0.949** 0.809* 0.391 -0.784 葡萄糖 -0.635 0.895* 0.716 0.319 -0.695 蔗糖 -0.866* -0.917* -0.473 0.870* 云南枸杞 果糖 0.999** -0.720 0.912* 0.629 -0.896* -0.366 葡萄糖 -0.727 0.918* 0.647 -0.891* -0.371 蔗糖 -0.811* -0.592 0.739 -0.311 中国枸杞 果糖 0.925* -0.531 0.713 -0.063 -0.721 -0.247 葡萄糖 -0.741 0.759 -0.367 -0.648 -0.249 蔗糖 -0.806* 0.634 0.204 0.646 说明*代表显著水平(P<0.05);**代表极显著水平(P<0.01) Table 2. Correlation coefficients between sugar content and activity of sucrose-metabolizing enzymes in 4 wolfberry species
2.1. 枸杞成熟期果实糖质量分数
2.2. 枸杞果实发育过程中果实糖质量分数变化
2.3. 枸杞果实发育过程中果实蔗糖代谢酶活性变化
2.4. 枸杞果实枸杞糖质量分数与蔗糖代谢酶活性的关系
-
糖分的积累是果实品质形成的关键,其糖种类与糖质量分数对果实风味和品质具有重要影响[17-18]。糖也是色素、氨基酸、维生素和芳香物质等其他营养成分合成的基础原料,还与其他重要的生理功能有关,如参与果实细胞膨大渗透推动力,调节植物生长发育过程中许多相关基因的表达[19-20]。本研究利用GC对不同种枸杞材料进行检测,共检测出10种糖,分别为果糖、葡萄糖、蔗糖、赤藓糖、阿拉伯糖、鼠李糖、岩藻糖、半乳糖、木糖和山梨糖,其中宁夏枸杞检测出10种糖,种类最多;云南枸杞和中国枸杞检测到7种糖,种类次之;北方枸杞检测到6种糖,种类最少。其结果与SUNG等[6]利用气相质谱联用法(GC-MS)在宁夏枸杞检测出11种糖和杨晓萍等[7]利用GC法检测到枸杞4种主要糖基本相一致,但因检测方法的差异,本研究在宁夏枸杞果实中没有检测到核糖。不同种间果实中糖种类与质量分数差异较大。宁夏枸杞是中国传统名贵中药材,至今已有500多年栽培历史[1],而另外3种枸杞近年来在中国一些地区零星栽植,其果实的苦涩味偏重(资料未发表),这与本研究测定果实甜度值相一致。枸杞果实糖种类与糖质量分数差异一方面取决于自身遗传特性,另一方面取决于栽培环境条件,此外,也可能与枸杞遗传进化也有关,有待于进一步研究。
本研究对不同种枸杞果实发育过程中果糖和葡萄糖质量分数的变化研究发现:不同种间果糖和葡萄糖质量分数差异较大,其中,在幼果期和青果期云南枸杞中未检测出葡萄糖,而中国枸杞2种糖质量分数较高;在初熟期和成熟期宁夏枸杞2种糖质量分数较高;在整个果实发育过程中,云南枸杞维持着较低果糖和葡萄糖质量分数。在宁夏枸杞上结果与前人的研究基本相一致[14, 21]。本研究发现不同种枸杞果实蔗糖质量分数呈现出不同变化趋势,其中,宁夏枸杞呈现出“降—升—降”趋势,从幼果期到色变期缓慢降低,之后缓慢升高,从初熟期到成熟期快速降低。郑国琦等[10]和冯美等[9]发现‘宁杞1号’蔗糖质量分数随着果实生长蔗糖质量分数逐渐增加,在花后31 d升至最高,随后又降低。其结果与本研究在宁夏枸杞上变化不一致。栽培环境、气候因素可能是造成本研究不同于前人结果的主要因素。
蔗糖是大多数植物同化产物运输的主要物质,也是许多果实中糖积累的主要成分[22]。蔗糖进入果实后,要经过一系列的酶代谢过程,在这些过程中起主要作用的有转化酶(IN)、蔗糖合成酶(SS)和蔗糖磷酸合成酶(SPS),最终将蔗糖转化成其他糖形式被植物体利用[23]。冯美等[9]发现宁夏枸杞果实转色前后枸杞果实中AI 和SPS的活性均明显升高,SS的活性明显降低;郑国琦[14]发现宁夏枸杞果实中的2种转化酶随着果实的发育呈现逐渐增大的趋势,SS呈现出先增加后下降趋势,而SPS变化幅度较小,整个果实发育过程中活性明显低于SS;张萍等[13]发现亏缺灌溉下枸杞果实全生育期转化酶活性保持在较高水平,但从转色期到成熟期呈下降趋势。本研究结果表明:不同种枸杞果实蔗糖代谢酶活性变化呈现不同变化趋势,其中宁夏枸杞转化酶活性总体呈现为逐渐升高的趋势,这与前人研究基本相一致。本研究中宁夏枸杞在果实发育前期SPS和SS的活性变化情况不同于前人研究,但在果实发育的后期AI和SS活性高于NI和SPS,这与郑国琦等[10]研究基本一致。说明宁夏枸杞果实发育过程中蔗糖代谢酶也存在时空差异。进一步分析果实糖质量分数与蔗糖代谢酶之间相关性表明:在枸杞果实发育过程中,3种枸杞果实中果糖质量分数与AI活性显著正相关,而郑国琦等[10]和冯美[12]也有相似的发现,这也说明转化酶在枸杞果实糖积累过程中发挥着重要作用。












 DownLoad:
DownLoad: